

Research center supported by FAPESP simplifies and cheapens the fabrication of glass-ceramic material that can be used in ocular prosthetics and dentin hypersensitivity treatment, among other applications (image: CeRTEV)
Published on 05/12/2021
By Elton Alisson in São Carlos (Brazil) | Agência FAPESP – A technique developed by researchers in São Carlos, São Paulo State, Brazil, promises to simplify and cheapen the fabrication of a bioactive glass-ceramic material with a wide array of potential applications.
The material can be used in ocular prosthetics, artificial ear ossicles, dental treatment and several other medical solutions, with less rejection than other implants and more stimulation of bone tissue regeneration.
Researchers at the Center for Research, Technology and Education in Vitreous Materials (CeRTEV), hosted by the Federal University of São Carlos (UFSCar), used 3D printing to produce structures with this highly porous glass-ceramic as a scaffold to induce bone regeneration.
CeRTEV is a Research, Innovation and Dissemination Center (RIDC) funded by FAPESP.
The new method, developed in collaboration with researchers at Italy’s University of Padua, Pennsylvania State University in the United States and Egypt’s National Research Center, is described in an article published in the Journal of the American Ceramic Society (JACS) and recently highlighted in a JACS special editorial feature.
“The study is at the forefront of relevant ceramics research and development. It provides insights into new ways to improve the sustainable manufacturing of glass-ceramics for biomedical applications,” said Jonathon Foreman, Managing Editor of JACS.
Starting from inexpensive commercial silicone resins, calcium and sodium carbonates, and disodium phosphate, the researchers produced compositions similar to Biosilicate, a material developed by CeRTEV in the 1990s, patented, and licensed to a private-sector company. It is now produced in the form of powder, granules, macroporous scaffolds for bone grafts, fibers, and monolithic (single) parts using conventional glass-ceramic fabrication technology, which consists of the controlled crystallization of a special glass by heat treatment.
“Ordinary glass is brittle. By means of controlled crystallization of bioglass, we were able to produce a material equivalent to Biosilicate that has high mechanical strength and is machinable, bioactive and bactericidal,” said Edgar Dutra Zanotto, a professor at the Federal University of São Carlos (UFSCar) and Director of CeRTEV.
When the material comes into contact with body fluids such as saliva or blood, it undergoes reactions that lead to the formation of a surface layer of hydroxyl carbonated apatite (HCA), a compound similar to the mineral fraction of natural bone. As a result, the bioactive glass-ceramic material adheres to bone, teeth and even cartilage, as well as stimulating bone tissue regeneration.
“Biosilicate has been recognized as an excellent material for applications in bone tissue engineering,” Zanotto said in a presentation to the first Symposium on Research and Innovation in Functional Materials held by the Center for Development of Functional Materials (RIDC CDMF) on May 23-24, 2019, at UFSCar.
To develop an alternative processing route for the material, the researchers subjected silicone-based polymers containing oxide microparticles as fillers to heat treatment, using this innovative process to make polymer-derived bioactive silicate ceramics.
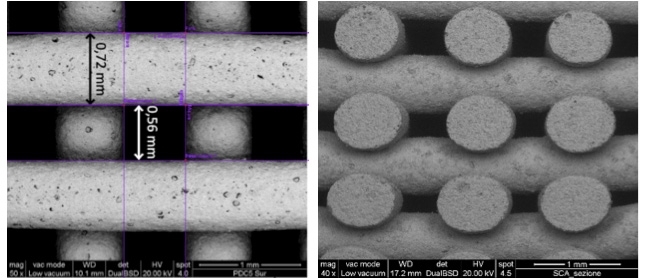
From silicone filaments containing active microparticles, they produced highly porous compositions similar to Biosilicate and then used 3D printing to create complex glass-ceramic scaffolds and molded foams.
The scaffolds and foams were fired at 1000 °C. Tests showed that the materials were highly porous (60%-75%) with compressive strength exceeding 7 megapascals (MPa), or 70 kg/cm², in the case of the scaffolds and 1.5-6 MPa, or 1.5-6 kg/ cm², for the foams.
“We demonstrated that preceramic polymers containing suitable oxide precursor fillers can be used for the direct synthesis of Biosilicate via a fast and simple route,” Zanotto said. “These materials have the potential to reduce raw material and processing costs, as well as reducing emissions of volatile organic compounds.”
Tissue engineering
Biosilicate produced by the traditional method was successfully tested in several in vitro experiments, in experiments with animals and in clinical trials involving a wide array of tissue engineering applications.
One was for the treatment of dentin hypersensitivity – acute pain triggered by hot or cold food and drink due to recession of the gums and exposure of dentin tubules.
The glass-ceramic material was applied as a powder comprising microparticles that filled the dentin tubules. In contact with saliva, the material underwent reactions leading to the formation of a surface coating of HCA, blocking the tubules and preventing their stimulation by cold or heat.
“A clinical study conducted with the material showed that six applications were sufficient to eliminate the patient’s dentin hypersensitivity,” Zanotto said.
Biosilicate is also used to make artificial middle ear ossicles, such as the hammer, anvil and stirrup. These are approximately 10 mm in length and 1-2 mm thick. They are implanted in patients with hearing loss due to problems in these ossicles caused by infection or disease.
In a clinical trial conducted by researchers at the University of São Paulo’s Ribeirão Preto Medical School (FMRP-USP), 24 out of 30 patients with this type of deafness recovered their hearing after ossicle implantation.
“Before ossicle implantation they could hardly hear anything,” Zanotto said. “After it they were able to hear sounds louder than a whisper, which is approximately 10 decibels.”
More recently, Biosilicate has been used in intraorbital implants (“artificial eyes”), which adhere to ocular tissue because the material is bioactive.
“These implants acquire the same mobility as a healthy eye, with the additional benefit that the material is bactericidal, minimizing the risk of infection,” Zanotto said.
The material used in conventional ocular prostheses has been banned by ANVISA, Brazil’s National Health Surveillance Agency, he added.
“There’s a huge market in Brazil for the ocular implant we’ve developed. We’ve licensed the material to a company, which is awaiting authorization from ANVISA to commercialize it,” Zanotto said.
The JACS article “Biosilicate scaffolds produced by 3D-printing and direct foaming using preceramic polymers” (DOI: 10.1111 / jace.15948) by Hamada Elsayed, Pietro Rebesan, Murilo C. Crovace, Edgar D. Zanotto, Paolo Colombo and Enrico Bernardo can be retrieved from ceramics.onlinelibrary.wiley.com/doi/full/10.1111/jace.15948.
Source: https://agencia.fapesp.br/30907