

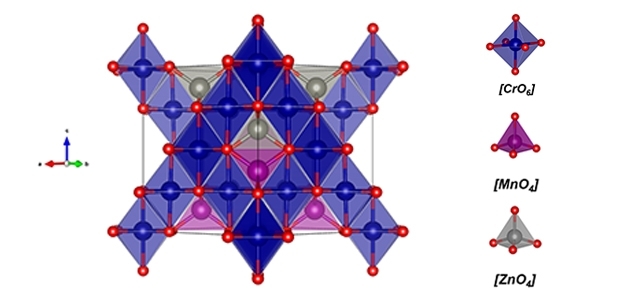
Thanks to its magnetic properties, the material – zinc-doped manganese chromite – can be used in a range of products, from gas sensors to data storage devices (structure of Mn0.5Zn0.5Cr2O4 molecule with corresponding atom clusters / image: Renan Ribeiro)
Published on 03/25/2021
By José Tadeu Arantes | Agência FAPESP – Spinels are oxides with chemical formulas of the type AB2O4, where A is a divalent metal cation (positive ion), B is a trivalent metal cation, and O is oxygen. Spinels are valued for their beauty, which derives from the molecules’ spatial configurations, but spinels in which the trivalent cation B consists of the element chrome (Cr) have aroused strong interest for a reason that has nothing to do with aesthetics: they have magnetic properties with an abundance of potential technological applications, from gas sensors to drug carriers, and from data storage media to components of telecommunications systems.
A study by Brazilian and Indian researchers investigated a peculiar kind of spinel: zinc-doped manganese chromite. Nanoparticles of this material, described by the formula Mn0.5Zn0.5Cr2O4 [where manganese (Mn) and zinc (Zn) compose the A-site divalent cation], were synthesized in the laboratory and characterized by calculations based on density functional theory (DFT), a method derived from quantum mechanics that is used in solid-state physics and chemistry to resolve complex crystal structures.
The material’s structural, electronic, vibrational and magnetic properties were determined by X-ray diffraction, neutron diffraction, X-ray photoelectron spectroscopy and Raman spectroscopy. A report of the study has been published in the Journal of Magnetism and Magnetic Materials with the title “Structural, electronic, vibrational and magnetic properties of Zn2+ substituted MnCr2O4 nanoparticles”.
The Brazilian scientists who participated in the study are affiliated with the Center for Research and Development of Functional Materials (CDMF), one of the Research, Innovation and Dissemination Centers (RIDCs) supported by FAPESP.
A paramagnetic-to-antiferromagnetic phase transition was established at 19 kelvin (-254.15 Celsius). Paramagnetic materials are attracted by an external magnetic field because their atoms or molecules each have one electron with an unpaired spin. Magnetic materials have several organized unpaired electrons, and the cumulative effect of these electrons produces magnetic attraction. In antimagnetic or antiferromagnetic materials, the spins of all the electrons are paired, so that for every spin-up electron, there is a spin-down electron. As a result, they do not respond perceptibly to the presence of a moderate external magnetic field.
“Our interest in this material is due to its magnetic properties,” Elson Longo, one of the authors of the study, told Agência FAPESP. Longo is a Professor Emeritus in the Chemistry Department of the Federal University of São Carlos (UFSCar) in the state of São Paulo, Brazil, and CDMF’s principal investigator.
“Conventional studies consider magnetic properties generically, from the standpoint of the system as a whole, whereas we’ve developed a quantum mechanical method to determine magnetic properties on the basis of the morphologies of the surfaces of a material’s crystal structure,” Longo said. “Even before synthesizing any material, we’re able to predict its magnetic properties theoretically. In this specific case, we expected the zinc to promote an increase in the surface with magnetic properties, and this did indeed happen.”
According to Longo, to be properly understood, a crystal should be considered on three different scales. “At a long distance, we have the entire crystal. At a short distance, we have the smallest possible cluster of atoms. At a medium distance, we have two or more clusters interacting. If a cluster is perfectly ordered, it won’t display paramagnetic behavior, let alone magnetic behavior, because for every spin-up electron, there will be an offsetting spin-down electron. However, if any change is made – if the chemical bond angles are altered, for example – then unpaired electrons may appear, and the material may become paramagnetic or even magnetic,” he said.
This disturbance can also occur as a result of medium-distance interactions. Magnetism, therefore, can be produced by changes at both short and medium distances. The same material can display different properties depending on variations in certain parameters, which has to do with how the material is synthesized.
“CDMF is conducting studies concentrating on identifying very cheap materials with bactericidal and fungicidal properties. One of the applications would be the production of packaging to extend the shelf life of food products. Another focus is the identification of inorganic materials with anticancer properties. A third line of research aims to find photodegradation materials capable of breaking down organic molecules and converting them into carbon gas and water. These materials could be used to clean up rivers contaminated by pollutants,” Longo said.
The article “Structural, electronic, vibrational and magnetic properties of Zn2+ substituted MnCr2O4 nanoparticles” can be retrieved from www.sciencedirect.com/science/article/abs/pii/S0304885319337928.
Source: https://agencia.fapesp.br/33029