

Image: Aljava Biotech
Published on 05/27/2024
By Roseli Andrion | Agência FAPESP – A major difficulty in cancer treatment with chemotherapy, targeted inhibitors or immunotherapy is choosing the best medication for each case. International protocols designed to help physicians choose the conduct considered suitable for advanced or metastatic cancer provide a range of options. Oncologists make their choice based on the specific features of the case and the patient’s clinical conditions, typically without knowing how the patient will respond to selected medications.
Tumors are often found to be resistant to medications during the treatment cycle. In such cases, it becomes necessary to change the medication, said Vilma Martins, a researcher in the field. “This causes delays and unnecessary toxicity from formulas that don’t work. It also means unnecessary costs for the healthcare system, as money has to be spent on medications that are ineffectual for the case in question. Making the right choice from scratch is far better,” she said.
The main reason is the lack of genetic markers that can be used to predict the response to chemotherapy. Moreover, even when genomics is used to choose specific targeted drugs, nothing can guarantee a satisfactory result. “So it’s essential to have tests that predict the response and let physicians choose the best therapeutic conduct for each patient,” she said.
To achieve this goal, researchers at Aljava Biotech, a startup based in São Paulo city (Brazil), and co-founded by Martins with Tiago Góss and Luciana Osaki, are developing a test that will provide more precise information to help oncologists choose the best therapeutic approach.
Because every individual is unique, physicians need personalized information as a basis for decision-making. “We aim to ensure that the solution can predict the most suitable treatment for each patient with a high degree of specificity and sensitivity,” Martins said.
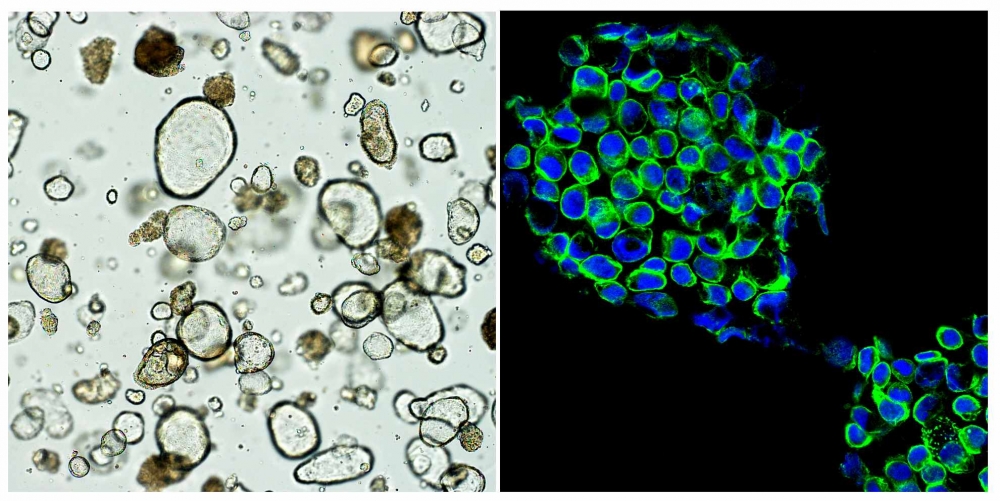
Organoids with tissue from patients
As research has evolved in this field, organoids – miniaturized 3D cell structures grown in the laboratory – have been found to provide a better way to mimic the patient’s organism than other methodologies. In the oncological field, tumor organoids, or tumoroids, are 3D models of tumors grown in vitro from cells taken from a patient’s tumor.
“When tumoroids are exposed to different medications, they can show how patients will respond to each one,” Martins explained.
To prove and apply this concept, Aljava’s scientists embarked on a study supported by FAPESP’s Innovative Research in Small Business Program (PIPE).
Led by Martins, the study was designed in collaboration with Góss at A.C.Camargo Cancer Center. “I left that hospital in 2021 to join the University of São Paulo’s Biomedical Sciences Institute [ICB-USP] as an associated researcher, and as we thought about ways of continuing the project, we decided to found a company. That’s how Aljava was born,” she recalled.
The goal is to establish functional tests using tumor cells from the patient. “They involve treating tumoroids with specific drugs that could be given to the patient, in order to find out which ones will be most effective. They could be conducted at the start of the treatment or when the disease progresses to a new stage, for example,” she said.
In a way, the scientists are attempting to determine the best way to hit the target, which is the disease. “Our test will enable patients to receive the treatment with the best chances of success,” she said.
The idea of a target is the key to the startup’s name: aljava is the Portuguese word for quiver. “We like to think of our technology as a ‘quiver’ full of ‘arrows’ that can be aimed at the tumor in a personalized manner,” she said.
Proof of concept
In setting out to develop the methodology, Martins was determined to make sure Aljava was linked to a cancer hospital, leading naturally to a partnership with A.C.Camargo Cancer Center. “We needed a large number of patients because the tests would have to be standardized. It was also essential to have a laboratory to help do the research and physicians interested in participating in the development of a model,” she said, adding that the infrastructure provided by both ICB-USP and A.C.Camargo Cancer Center has been very important to enable her and her collaborators to conduct the research.
They are now working on a proof of concept. “The first step is to identify patients with metastatic or advanced-stage cancer who have donated samples of tumor tissue. Next, the oncologists recruit volunteers, who consent to the development of tumoroids from their tissue and agree to be treated with different medications, which the oncologists are entirely free to choose. After the treatment cycles are completed, the patients’ responses are measured and compared with the responses of the tumoroids. The test’s predictive value is the outcome of this analysis,” Martins explained.
The team began the project with gastrointestinal tumors, which are the most prevalent. “Lab results correlated highly with patient treatment outcomes, and we were then able to bring the test to market,” Martins said.
The researchers have experience with tumoroid technology and can scale up the experiments to include more patients. “We aim to have the product available by end-2025,” she said.
In addition to FAPESP’s support for the project, Aljava Biotech receives funding from MKM Biotech, an angel investor. “They’ve provided financial support and helped us structure the firm,” she said.
In order to be considered successful, the test has to be completed within three weeks. “This is the lead time considered by oncologists if the onset of treatment is not to be compromised,” she said. The time constraint is a challenge, but for Martins the difficulties are the same as for any researcher involved in a project of this kind.
“The main challenge is tumor cell growth, which may be too slow or may not happen at all. This can influence the lead time to deliver results or prevent the test’s feasibility itself,” she said.
Once completed, the technology will be useful for different types of tumor. “While there are differences in the culture of each kind of tissue, the method can be deployed for any tumor, including non-solid cancers [such as leukemia and lymphoma],” she said.
Having this type of technology in Brazil is essential to avoid the high risk associated with the logistics of transporting samples between countries, and also to assure better experiences for all those involved, she continued. “The samples are made up of living cells that have to be processed within 24 hours. Imports are logistically complex, making the product expensive,” she said.
Other possibilities
Applications of the technology Aljava is developing will extend beyond determining the best treatment for cancer patients to deployment by the pharmaceutical industry in preclinical trials of new drugs. “Drug discovery and approval is time-consuming and costly. The models now in use have limitations that include the difficulty of reproducing them in clinical trials with patients,” Martins said.
In the United States, the use of tumoroids in preclinical trials has been authorized by the Food and Drug Administration (FDA). “Tumoroid biobanks have been created with material donated by patients, enabling the pharmaceutical industry to test novel compounds using models that predict patient responses more reliably. Aljava aims to establish and win approval for a biobank that offers this drug testing service to pharmaceutical companies and academic researchers,” she said.
Source: https://agencia.fapesp.br/51780