

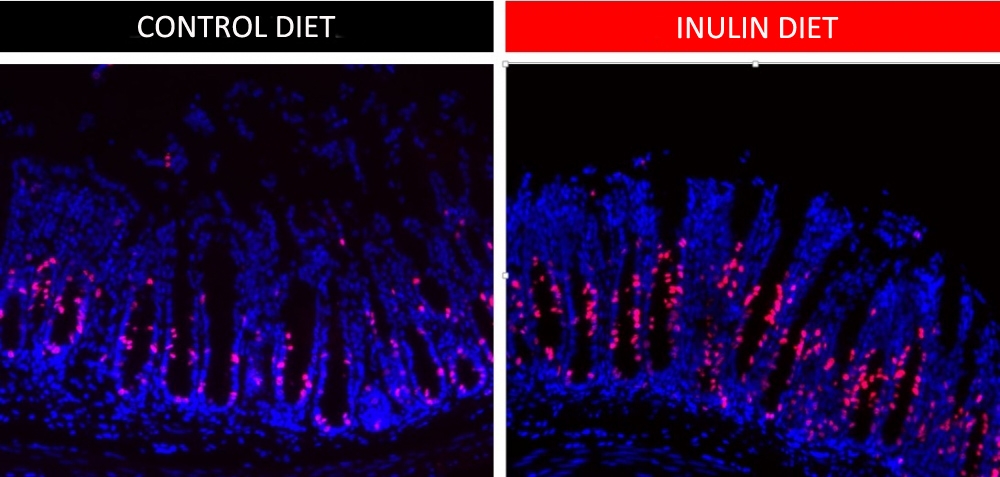
These images show more cells undergoing cell division (red) in the group fed an inulin-rich diet (right) than the group that did not ingest this soluble fiber. The experiment shows that proliferative activity increases in gut epithelial cells when soluble fiber is consumed (credit: Renan Corrêa)
Published on 07/24/2023
By André Julião | Agência FAPESP – A high-fiber diet is known to have several health benefits. Scientific studies point to protection against such diseases as cancer and diabetes. However, exactly how fiber interacts with the intestines and the microorganisms that colonize them – the gut microbiota – is poorly understood.
A study conducted by Brazilian researchers and reported in an article published in the journal Microbiome shows that a diet containing suitable amounts of inulin, a soluble fiber abundant in chicory roots, for example, can influence even the length and other macroscopic characteristics of the intestines.
This beneficial action is possible only in the presence of bacteria that digest the fiber, however. Positive alterations in immunity are among the healthy interactions. The findings include new evidence of the importance of dietary fiber and contribute to the medical understanding of inflammatory bowel disease.
“In mice that ingested a 10% inulin diet, the gut was larger than in mice that consumed only insoluble fiber [cellulose]. When we analyzed their intestinal tissue, we found more epithelial stem cell proliferation in the animals fed an inulin-rich diet. The epithelium is the layer that separates gut contents from other organs,” said Renan Oliveira Corrêa, first author of the article. He conducted the investigation with support from FAPESP during his PhD research at the State University of Campinas’s Institute of Biology (IB-UNICAMP) in São Paulo state.
Part of the analysis was done while Corrêa was a research intern at the Massachusetts Institute of Technology (MIT) in the United States. The study was part of a project led by Marco Aurélio Ramirez Vinolo, a professor at IB-UNICAMP and last author of the article.
Gene expression
The researchers sequenced RNA from the gut epithelium and detected 268 genes expressed differently by mice fed the inulin-rich diet and mice fed a normal diet. In the former group, expression of genes associated with the cell cycle and with DNA replication and repair was augmented. These cellular processes are extremely important because of the high rate of renewal of epithelial cells in the intestines.
On the other hand, genes associated with lipid and fatty acid metabolism were less expressed in the mice that consumed inulin. This modulation was compatible with effects described by other research groups in studies showing that inulin consumption lowers lipid levels in the bloodstream and reduces hepatic steatosis (fatty liver disease).
The inulin-rich diet also increased expression of genes associated with gut epithelial cell differentiation, a process essential to growth of the organ and substitution of dead cells. This led to an increase in the number of goblet cells, specialized epithelial cells that secrete mucins, which are significant components of the mucus that protects the human gut from harmful bacteria. Their abundance shows that the organ is in a healthy condition.
“This part of the study included a single-cell sequencing assay, possibly the first performed entirely in Brazil. The method measures the individual expression profiles of every cell in the epithelial layer,” Vinolo explained.
Vinolo also led previous research projects on the benefits of dietary fiber (read more at: agencia.fapesp.br/35523).
The role of bacteria
Gut microbiota was drastically modified in the inulin-fed mice. To make sure the alterations were relevant to inulin’s effects on the epithelium, the researchers conducted experiments involving two other groups of mice. Before ingesting inulin, one group was given an antibiotic that depleted its gut microbiota. The other group consisted of germ-free mice with no gut microbiota at all – they had been bred in a totally sterile environment and had no contact with any source of microorganisms. After ingesting inulin, neither group developed the epithelial proliferative phenotype, and neither displayed the molecular alterations observed in the group with normal gut microbiota.
“When they ingested a small amount of feces [via fecal transplant] from the inulin-fed mice with gut bacteria, these mice developed the phenotype of interest even though they themselves had never ingested inulin, confirming the key role of gut microbiota in these processes,” said Corrêa, who is currently a researcher at Imagine Institute in Paris, France.
Finally, the study showed that inulin consumption raised blood levels of interleukin-22 (IL-22), a cytokine produced by the immune system and important to intestinal health. In mice that did not produce IL-22 because the gene responsible for this protein had been silenced, the inulin-rich diet did not have the effects observed in the other groups.
Nor did inulin produce the effects of interest in mice lacking gamma delta T cells, a lymphocyte subgroup present in the epithelial layer of mucosa, pointing to an important function of specific immune cells in this context.
“We found that other soluble fibers, such as pectin from fruit, had similar effects. More research is needed to understand exactly what each fiber does, but we can state right now that the benefits of a balanced diet are increasingly evident and involve complex interactions between dietary components, gut microbiota and various types of cells,” Vinolo said.
“It’s crucial to understand how this system works and how we can act on it so as to prevent and even treat inflammatory bowel diseases, as well as others such as diabetes and asthma.”
The article “Inulin diet uncovers complex diet-microbiota-immune cell interactions remodeling the gut epithelium” is at: microbiomejournal.biomedcentral.com/articles/10.1186/s40168-023-01520-2.
Source: https://agencia.fapesp.br/41957