

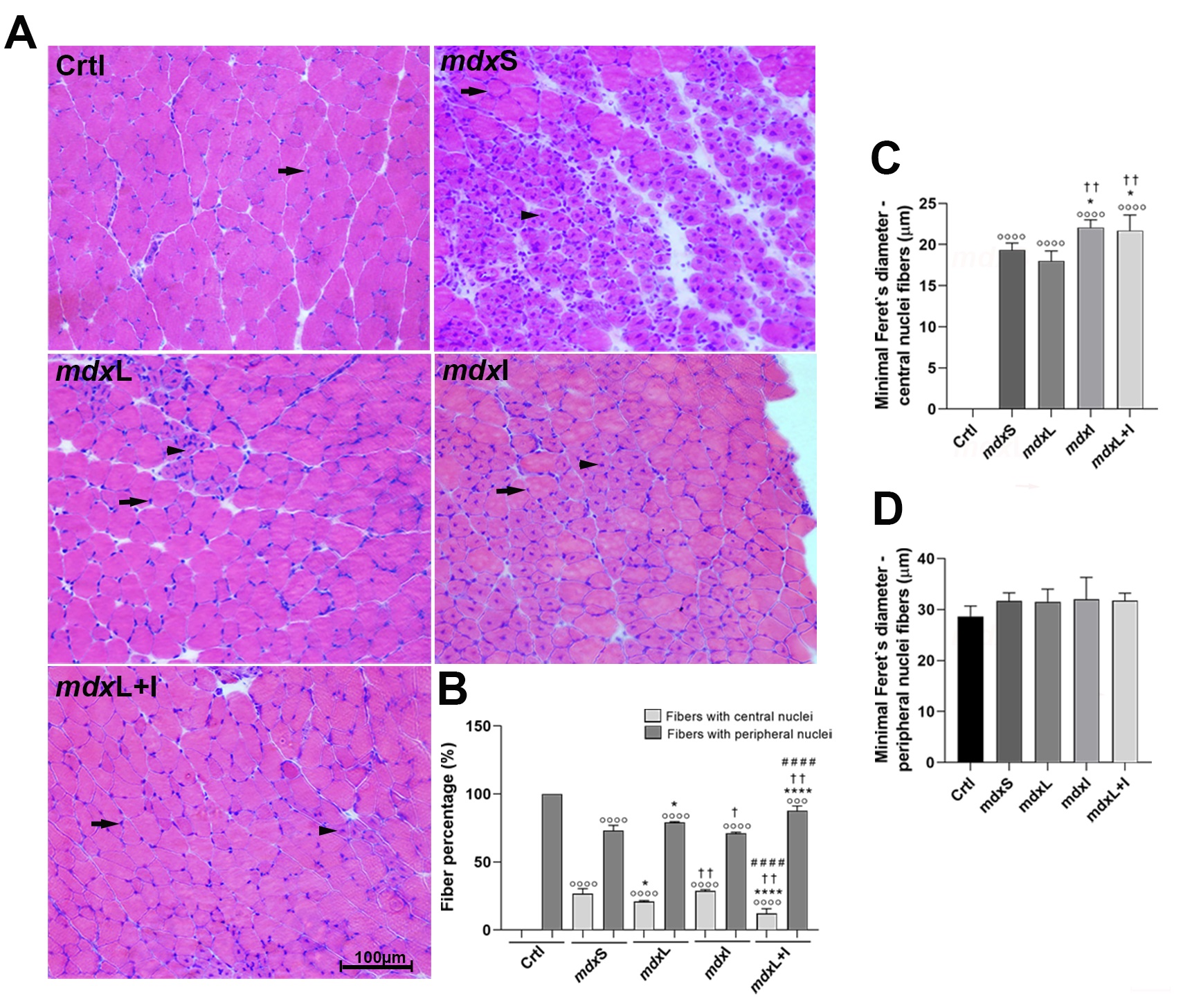
Representative quadriceps cross-sections show normal muscle fibers (black heads) and regenerated muscle fibers (black arrowheads) from each of the five experimental groups (image: Heloina Nathalliê Mariano da Silva et al./PLOS ONE)
Published on 06/10/2024
By Thais Szegö | Agência FAPESP – In an animal model of Duchenne muscular dystrophy, Brazilian researchers tested a therapy that combines photobiomodulation using laser light or light-emitting diodes (LEDs) with idebenone, an antioxidant compound investigated for application in neurodegenerative diseases.
As reported in an article published in the journal PLOS ONE, the strategy prevented muscle degeneration and improved regenerative capacity in the muscle fibers affected by the disease.
Duchenne muscular dystrophy is the most common and most severe form of childhood muscular dystrophy and most often affects boys. It is an incapacitating and lethal genetic disorder characterized by progressive muscle degeneration and weakness. It is estimated to affect one in 3,000 to 6,000 live male births.
Duchenne is caused by mutations in the gene that encodes dystrophin, a protein essential to muscle function. In skeletal muscle, dystrophin acts as a cytoskeletal stabilization protein and protects cells against contraction-induced damage. When dystrophin is absent or reduced, muscles cannot repair themselves or function properly. In Duchenne, contractile tissue is gradually replaced by fatty fibrotic connective tissue, causing inflammation and muscle wasting.
The earliest symptoms typically appear around 2-3 years of age. The disease affects both the voluntary muscles that control movement in the arms, legs and trunk but in the long term also weakens involuntary muscles, such as those that keep the heart beating and the lungs breathing.
Despite scientific advances in genetic and cellular therapy, no cure for this dystrophy has been found to date. The most widely used treatment involves administering glucocorticoids, a type of steroid hormone, but prolonged use has severe side effects such as hyperglycemia and bone loss leading to stunting of growth and retarded skeletal development.
“For these reasons, our research group has been focusing on studies of muscle fiber biology in dystrophin-deficient mice to seek novel therapies with the potential to minimize progression of the disease and improve the dystrophic patient’s quality of life,” said Professor Elaine Minatel, Vice Head of the Department of Structural and Functional Biology at the State University of Campinas’s Institute of Biology (IB-UNICAMP).
The photobiomodulation-idebenone combination is one of the novel therapies studied by the group. “Photobiomodulation, which is widely used in dermatology and orthopedic physical therapy, induces a photochemical process that stimulates the production of energy to boost cellular metabolism and produce such effects as tissue regeneration, wound healing, and reduction of muscle inflammation and fatigue,” Minatel explained.
Idebenone is a synthetic analog of coenzyme Q-10 with clinical applications in many central nervous system degenerative diseases. It was developed for treatment Alzheimer’s and is available generally as an antioxidant, but clinical trials have shown that it can improve respiratory function in dystrophic patients.
In light of the action mechanisms involved, the research group set out to see whether their combined use had a potential therapeutic effect on the muscle fibers of dystrophin-deficient patients by means of in vitro and in vivo experiments with mice.
For the in vitro study, dystrophic muscle cells were developed from limb muscles of 28-day-old mice and divided into four groups: untreated cells used as controls; cells treated with LED therapy; cells treated with idebenone; and cells treated with both in combination.
For the in vivo study, normal untreated mice served as controls and the dystrophic mice were divided into four groups: sham LED therapy plus carboxymethylcellulose sodium salt diluted in water; idebenone diluted in carboxymethylcellulose sodium salt; LED therapy; and LED therapy combined with idebenone.
According to Minatel, the results showed that LED therapy and idebenone were beneficial when administered singly or combined. “The combination displayed synergistic effects on some specific parameters, but in most respects, the results were similar whether each was administered alone or in combination with the other,” she said.
“They were administered in accordance with parameters calculated specifically for the experimental model, such as dosage and timing. The treatments modulated the autophagic process that leads to a breakdown of cellular components and improved the regenerative capacity of muscle fibers.”
Photobiomodulation and idebenone, individually or together, do not replace the standard pharmacological treatment with glucocorticoids, she stressed. “Another point that should be made is that the results obtained in an experimental model won’t automatically be reproducible in human patients, for several reasons, such as differences in the severity of the muscles affected in dystrophic patients and mdx mice [which mimic Duchenne], and progression of the disease, among others,” she said, adding that clinical trials will be needed to investigate the potential benefits of these therapies and their action mechanisms in dystrophic patients.
The article “LEDT and Idebenone treatment modulate autophagy and improve regenerative capacity in the dystrophic muscle through an AMPK-pathway" is at: journals.plos.org/plosone/article?id=10.1371/journal.pone.0300006.
Source: https://agencia.fapesp.br/51908