

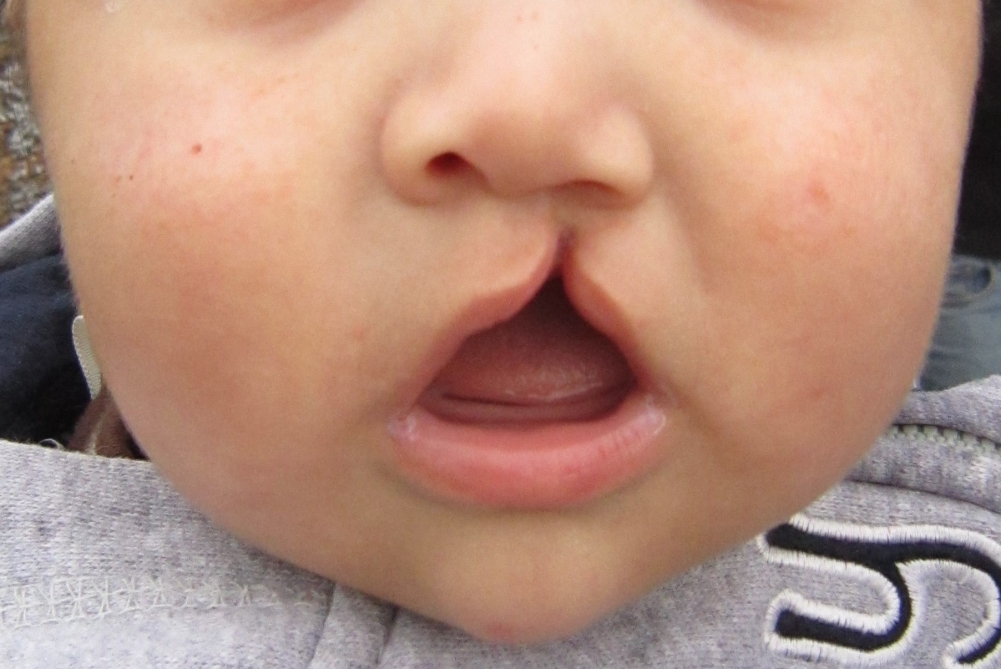
According to the research, in pregnant women with pro-inflammatory conditions, molecules called cytokines induce hypermethylation of the gene that contains in one of its alleles the mutation responsible for the occurrence of cleft lip in the offspring (photo: James Heilman/Wikimedia Commons)
Published on 05/17/2024
By Maria Fernanda Ziegler | Agência FAPESP – Researchers from the University of São Paulo (USP), in Brazil, and University College London (UCL), in the United Kingdom, have demonstrated in an animal model that cleft lip and palate is due to the association of two factors: one genetic and the other associated with the occurrence of inflammation during pregnancy – during the period of formation and development of the embryo.
In an article published in the journal Nature Communications, the group describes how the gene-environment relationship can cause craniofacial malformations. The study was conducted at the Human Genome and Stem Cell Research Center (HUG-CELL), a FAPESP Research, Innovation and Dissemination Center (RIDC) based at the Institute of Biosciences (IB-USP).
“Our group has been following families with cases of cleft lip for years, and there was a suspicion that there had to be an environmental as well as a genetic component for the malformation to occur. When we did the genetic sequencing of these people, we saw that although many of them had the mutation in the CDH1 gene, a significant proportion did not have the malformation. What was missing was a piece that fully explained what led to the occurrence of cleft lip,” says Maria Rita Passos-Bueno, the HUG-CELL researcher who coordinated the study.
Another element that supports the hypothesis, according to Passos-Bueno, is that there is a wide variation in the severity of clefts among the people being monitored. “People with the same genetic mutation may have no cleft lip, only an opening in the lip or the roof of the mouth, or even have both the lip and the roof of the mouth affected,” she says.
The researcher explains that when there is a mutation in one of the alleles of the CDH1 gene (which codes for the E-cadherin protein), the result can be the development of cleft lip and also a type of gastric cancer. The mutation interferes with the migration process of neural crest cells – cells present during embryonic development that differentiate to form bones, cartilage and connective tissue of the face, among other cell types.
If the migration of neural crests is compromised during embryonic development, the differentiation process is impaired, which can result in cleft lip.
However, Passos-Bueno points out that this variant alone does not fully explain the hereditary problem of cleft lip. “When both alleles of CDH1 are affected, the embryo dies. When one allele is normal and the other is mutated, there’s compatibility with life and, in most cases, no malformation occurs. This phenomenon of heredity is called incomplete penetrance: the genetic mutation exists, but it isn’t translated into the phenotype,” explains Passos-Bueno.
In their search for the missing piece of the puzzle, the researchers began to investigate possible environmental factors that might contribute to the malformation process.
“Data from the cleft lip population show that obesity, diabetes and other pro-inflammatory situations, such as maternal infections [episodes of fever during pregnancy], are risk factors for children being born with clefts. But we needed to show how this relationship occurred. The results of our study showed that inflammatory molecules called cytokines [secreted by cells of the immune system] induce hypermethylation of the CDH1 gene [a biochemical modification that alters the gene’s expression pattern],” says Passos-Bueno.
This is the phenomenon that scientists call epigenetics, i.e. biochemical changes in cells caused by environmental stimuli (in this case inflammation) that promote the activation or silencing of genes without causing changes in the genome (mutations).
“The challenge of the study was to show this relationship between genetics and the environment, i.e. the factor that specifically or primarily leads to methylation of the CDH1 gene in a pregnant woman carrying the hereditary mutation who has some stage of pro-inflammation during pregnancy,” says Lucas Alvizi, who was a FAPESP postdoctoral fellow at the time of the research. Alvizi is now a researcher at UCL.
It should be noted that methylation is a biochemical modification that consists in the addition of a methyl group to the DNA molecule through the action of enzymes. This is a natural and necessary process for the functioning of the organism, through which the expression of genes is modulated. However, when this process is deregulated – as in the case of CDH1 hypermethylation – it can cause cellular dysfunction and contribute to the development of diseases and malformations.
In addition to in vitro tests with human cells, the researchers conducted experiments with mice and frogs to prove that inflammation caused hypermethylation in the CDH1 gene. They found that adding a methyl group to a specific stretch of DNA impeded the gene’s transcription process, resulting in less neural crest migration.
“We exposed the cells and embryos of mice and frogs with a mutation in one of the alleles of the CDH1 gene to environmental factors, in this case, bacterial particles that induce inflammation. We then took females with normal copies of the gene and exposed them to the same condition. And we observed that only the offspring of the females carrying the mutation had defects in the migration of the neural crest, which could explain the appearance of the cleft,” reports Alvizi.
Passos-Bueno explains that mutations in the CDH1 gene can also lead to a type of hereditary stomach cancer. “Recent studies have shown that some families had both clefts and cancer, but this is rarer. In future studies, we intend to investigate the relationship between these two factors [genetic and environmental] and stomach cancer,” she says.
Another future goal of the team is to understand which pro-inflammatory states during pregnancy, associated with the genetic makeup of the embryo, are sufficient to cause cleft lip. In Passos-Bueno’s opinion, this knowledge could guide actions capable of preventing the malformation.
The study “Neural crest E-cadherin loss drives cleft lip/palate by epigenetic modulation via pro-inflammatory gene-environment interaction” can be read at: www.nature.com/articles/s41467-023-38526-1.
Source: https://agencia.fapesp.br/51700