

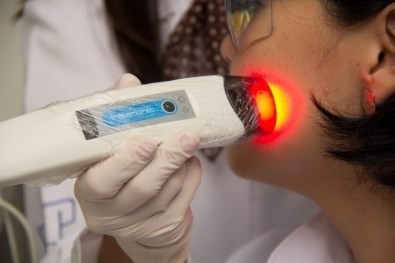
Photodynamic therapy developed by the Center for Research in Optics and Photonics, which is supported by FAPESP, has been recommended by Brazil’s federal body responsible for including novel healthcare technologies in the national health service (image: CEPOF)
Published on 08/07/2023
By Elton Alisson* | FAPESP Innovative R&D – A photodynamic therapy that offers an alternative method of diagnosing and non-invasively treating basal cell carcinoma, the most frequent type of skin cancer worldwide, could soon be available from public health services and free at the point of delivery in Brazil.
An arm of the Brazilian Health Ministry called CONITEC recently recommended that the therapy be provided by the SUS (Sistema Único de Saúde, similar to the UK’s NHS).
The technique, which involves a novel device that is cheap and easy to make, was developed by researchers affiliated with the Center for Research in Optics and Photonics (CEPOF), one of the Research, Innovation and Dissemination Center (RIDCs) funded by FAPESP. CEPOF is hosted by the University of Sao Paulo’s São Carlos Institute of Physics (IFSC-USP).
“The recommendation of delivery by the SUS is a signal achievement for IFSC-USP. If it happens, Brazil will be the first country to offer photodynamic therapy free of charge via public health services, as far as we know. This outcome demonstrates the importance of funding basic and applied research, as FAPESP does via its RIDCs, which contribute to the development and implementation of novel healthcare technologies,” said Cristina Kurachi, a professor at IFSC-USP and one of the authors of the technique.
Developed with the support of FINEP, the Brazilian Innovation Agency, an arm of the Ministry for Science, Technology and Innovation, and made by MM Optics, a startup based in São Carlos, the device uses optical fluorescence to recognize the edges of skin cancer lesions in minutes. The lesion is then treated with a methyl aminolevulinate (MAL) cream. MAL is a derivative of 5-aminolevulinic acid (5-ALA). After three hours of contact with the skin, the compound is absorbed and, inside the tumor cell mitochondria, gives rise to protoporphyrin, a photosensitizing pigment that is a precursor of chlorophyll.
The cream is removed from the lesion, and the region is irradiated for 20 minutes with a 630-nanometer red light source coupled to the device. The light activates the protoporphyrin and triggers a sequence of reactions in the tumor cells, generating reactive oxygen species capable of eliminating the lesions while leaving healthy tissue intact.
The device produces fluorescence images after the procedure to ensure total irradiation of the lesions. Two sessions are required for the treatment, with a one-week interval between sessions.
Clinical trials were held to validate the technique at 72 health clinics nationwide, via a project supported by BNDES, the national development bank, and FINEP. The multicenter study was led by Vanderlei Salvador Bagnato, a professor at IFSC-USP and CEPOF’s principal investigator.
At Hospital Amaral Carvalho in Jaú, São Paulo state, for example, more than 2,000 lesions were treated with the new technique, and 40 groups of physicians were trained to use it. Clinical trials were also conducted in nine other Latin American countries.
The results of the clinical trials showed that the treatment eliminated about 85% of the tumors treated without scarring or side effects except for minor redness at the site.
A new protocol developed by the group entails two applications in a single clinical session, eliminating 93% of tumors.
“A great many patients have to travel a long way in order to get access to surgical treatment or live in cities with long waiting lists. In these cases, photodynamic therapy administered as an outpatient procedure is a significant alternative option. Moreover, it’s safe and effective,” Kurachi said.
Promising results
According to a press release from CONITEC, this was the first application from a university for a license to have the SUS deliver a healthcare solution and a successful case of technological innovation by Brazilian scientists.
“Brazilian universities play a central role in innovation, recognizing the needs of the SUS, fostering research, development and innovation, producing clinical evidence, training and equipping health services, and participating in the inclusion and delivery of novel technologies by the SUS,” said Luciene Bonan, head of the Health Ministry’s Department of Technology Management and Inclusion (DGITS).
The application for inclusion was submitted by the University of São Paulo (USP). The Federal University of São Paulo (UNIFESP) analyzed the application on behalf of the ministry. UNIFESP is a member of the Brazilian Health Technology Evaluation Network (REBRATS).
On June 28, CONITEC ended a public consultation on the application, concluding that the therapy had worked well for patients with basal cell carcinoma and had been shown to be safe and effective in cases where surgical intervention was not recommended.
Basal cell carcinoma is the most common type of skin cancer and the most frequently diagnosed type of cancer generally. The first line of treatment is surgical removal, but CONITEC noted that photodynamic therapy is best for patients who cannot undergo surgery or whose tumors are considered low-risk, as it is an outpatient procedure and does not require sophisticated infrastructure. The final recommendation was inclusion by the SUS since qualified professionals and the necessary equipment already exist in many public health clinics.
The treatment also proved effective in the long term, according to samples from clinical trials conducted by the Brazilian researchers at renowned cancer centers, showing extremely low relapse rates after treatment with photodynamic therapy and a cure rate of 90%.
CONITEC recommended inclusion of the technology on the basis of the Health Ministry’s approval procedures. The final decision will be made by the ministry’s science, technology, innovation and health complex secretary (SECTICS) and published in the federal gazette.
* With information from CONITEC.
Source: https://agencia.fapesp.br/42070