

A webinar held by FAPESP featured researchers from Brazil and Germany whose findings offer clues as to how SARS-CoV-2 invades the central nervous system and which cells are most affected (experiments with nerve cells conducted by researchers at D’Or Institute and UFRJ; reproduction)
Published on 07/27/2021
By Maria Fernanda Ziegler | Agência FAPESP – Days after the World Health Organization (WHO) declared COVID-19 to be a pandemic, in March 2020, a study of patients in Italy reported loss of smell and taste as a symptom. The first study to analyze the neurological impact of the disease in hundreds of people was published in April. Since then, research into the consequences of COVID-19 for the brain has discussed the effects observed in the acute stage, and neurological complications reported by some 30% of patients who recover.
“COVID-19 was initially described as a viral infection of the respiratory tract, but we soon learned that the brain is one of several affected organs. Some aspects of the disease remain obscure. The impact on the brain isn’t fully understood. It’s very important to stimulate the sharing of knowledge and experience among researchers around the world,” said Luiz Eugênio Mello, Scientific Director of FAPESP, in his welcoming remarks to the online seminar “What does COVID-19 have to do with the brain?”, held on July 7. The webinar featured speakers from Brazil and Germany and was the latest in the series FAPESP COVID-19 Research Webinars, organized with the support of the Global Research Council (GRC).
Viral entry route
One of the studies presented to the webinar was conducted at Charité University of Medicine Berlin (Germany) and showed that the novel coronavirus uses the olfactory mucosa as the main route to enter the brain. “This is due to the anatomical proximity between the cells of the mucosa and the blood vessels and nerve cells in the area. Once the virus has entered the olfactory mucosa, it appears to use neuroanatomical connections like the olfactory nerve to reach the brain,” said Helena Radbruch, a researcher in the Department of Neuropathology who analyzed samples from olfactory mucosa and four brain regions of 33 patients who died from severe COVID-19.
Radbruch and her team tracked 180 other patients from the acute stage of the disease until months after they recovered. “The good news, especially for those who have had COVID-19, is that the virus doesn’t stay in the brain. We found it there only in some cases, and it was no longer there a month or two after the acute stage,” she said.
They also found clear evidence of activated immune cells in the brain, medulla, and other parts of the central nervous system. In some cases, there was tissue and neuron damage due to hypercoagulation and acute infarction.
“The entry of the virus into the central nervous system via the neurons of the olfactory mucosa and other cranial nerves appears to explain neurological symptoms such as loss of smell and taste, which isn’t at all rare among COVID-19 patients,” she said.
In Brazil, researchers at D’Or Institute (IDOR) and the Federal University of Rio de Janeiro (UFRJ) conducted a series of experiments and concluded that the virus enters the brain by other routes besides the olfactory mucosa, including systemic inflammation as the disease progresses and affects different organs.
“Unfortunately, in one autopsy we found severe viral infection of the choroid plexus, a central nervous system structure protected by the blood-brain barrier. This brain region has a large amount of ACE2, the protein to which the virus binds to invade the organism. ACE2 is also abundant in the lungs,” said Marilia Zaluar Guimarães, a researcher at UFRJ and IDOR.
The study arose from the rare case of a one-year-old infant who already suffered from encephalopathy and did not survive COVID-19. The autopsy revealed the presence of viral particles in the lungs, heart, cortex and choroid plexus. “Infection by SARS-CoV-2 caused pneumonia, meningitis and damage to multiple organs owing to thrombosis, including the kidneys, lungs, brain, heart and pancreas,” she explained.
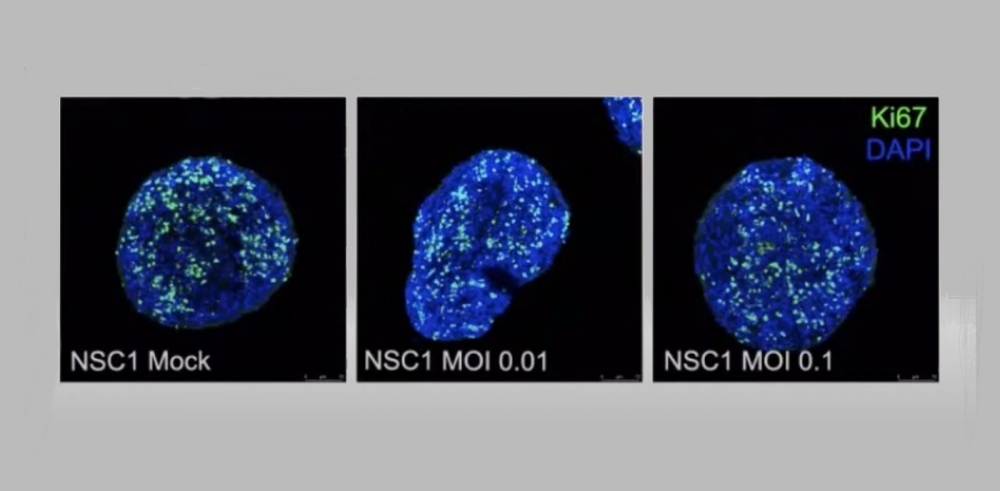
Having confirmed that the virus can breach the blood-brain barrier and infect the brain, the researchers decided to conduct studies with brain organoids, highly simplified genetically engineered models of the brain, and laboratory-cultured neural precursor cells called neurospheres. These were originally developed during the zika epidemic using induced human pluripotent stem cells (hIPSCs), skin or blood cells reprogrammed to return to a stage similar to stem cells. The hIPSCs are stimulated to differentiate into nerve cells such as neuroprogenitors, astrocytes and neurons.
“The organoids are a very simplified model of the brain, but they contain several cell types that let us study the progression of viral infection. In this manner, we proved that SARS-CoV-2 causes brain damage but isn’t able to replicate in the brain since three days after infection there was no viral presence in the neurospheres, which was puzzling,” Zaluar said. The researchers also found that infection reduced the number of neuroprogenitors but did not prevent these cells from proliferating.
Similar studies conducted previously had detected viral replication in brain organoids and neurospheres, but used much larger quantities of the virus and exposed the cell cultures for 24 hours (versus one hour in her study), she noted, concluding that systemic events could explain most of the neurological damage seen in severe COVID-19 patients, although the virus also causes direct brain damage and neuroinflammation.
The research presented by both Zaluar and Radbruch showed that although the virus is cleared from the brain a short time after infection, the elevated levels of pro-inflammatory cytokines persist well after the acute stage, which probably explains the neurological problems typical of long COVID.
Glial cells
Scientists affiliated with the University of Campinas (UNICAMP) and the University of São Paulo (USP) conducted a study, with support from FAPESP, in which they analyzed data for 81 subjects who tested positive for COVID-19 but had mild symptoms or none and did not require hospitalization. More than 50 days after they were diagnosed, they were found to have alterations in the structure of the cerebral cortex associated with the olfactory tract. In addition, 28% suffered to some degree from anxiety, 20% from depression, 28% from memory loss, and 34% from cognitive impairment.
The researchers also analyzed brain tissue samples from 26 patients who died after contracting COVID-19. The presence of the virus was confirmed in all samples, and alterations that suggested damage to the central nervous system were detected in five samples.
“We already knew of such neurological symptoms as loss of smell and taste, but our studies showed for the first time that the virus infects and replicates in astrocytes, which are the most abundant cells in the central nervous system and essential to the maintenance of neurons,” said Marcelo Mori, a professor at UNICAMP’s Institute of Biology (more at: agencia.fapesp.br/34404).
Researchers who collaborate via the Instituto Pasteur-USP scientific platform highlighted another interesting aspect of the effects of COVID-19 on the brain. Metabolic alterations in infected glial cells (astrocytes and other cell types that support and nourish neurons) may be related not only to the effects of the disease on the brain in the acute stage of the disease but also to long-term neurological complications reported by some patients.
“Animal model studies show that the virus can infect glial cells, where it can replicate, produce new viral copies and cause structural changes that affect cell metabolism,” said Jean Pierre Peron, a researcher at USP’s Biomedical Sciences Institute (ICB) and principal investigator for a project on the topic supported by FAPESP.
The scientists conducted analyses to verify alterations in protein expression by infected cells (proteomics) and in the metabolism (metabolomics). “We found many alterations in protein expression, especially those involved in carbon and glucose metabolism,” Peron said. “Not by chance, these signaling pathways are associated with brain disorders such as Huntington’s, amyotrophic lateral sclerosis and long-term depression.”
The metabolomic analysis showed hyperactivation of glycolytic pathways and mitochondria in infected glial cells. “Overall, we found that during infection by the virus there were changes in protein expression that correlated with the carbon metabolism of these glial cells,” Peron said, noting that the alterations in the expression of the enzyme glutaminase are probably associated with viral replication. “The enzyme is extremely important to glial cells, as 90% of the synapses in the brain are glutamatergic [mediated by the neurotransmitter glutamate]. We believe the virus needs glutamine to replicate in the brain and release fully mature particles. When glutaminase is blocked, the release of pro-inflammatory cytokines is reduced,” he explained. Glutaminase is a mitochondrial enzyme that catalyzes the breakdown of glutamine to form glutamate as a part of the glutaminolysis pathway. The glutamate system is important for information processing in neuronal networks.
A recording of the complete webinar can be watched at: covid19.fapesp.br/o-que-covid-19-tem-a-ver-com-o-cerebro/550.
Source: https://agencia.fapesp.br/36437