

Research conducted by the Center for Research on Redox Processes in Biomedicine resulted in the detection of peroxymonocarbonate for the first time in human cells (figura: Chem. Res. Toxicol 2024)
Published on 09/17/2024
Agência FAPESP* – High levels of carbon dioxide (CO2) in the atmosphere can alter not only the climate of our planet but also the functioning of our cells. The gas interacts with hydrogen peroxide (H2O2), which performs various functions in the human body, giving rise to a potent oxidant called peroxymonocarbonate.
"More and more evidence is emerging that peroxymonocarbonate is important in both cells’ adaptive responses via redox signaling and in cellular dysfunction. There is also epidemiological evidence that the levels of CO2 our cities are close to reaching cause a number of physiological problems. And the mechanisms underlying the toxicity of CO2 are poorly understood,” said Ohara Augusto, full professor at the University of São Paulo’s Chemistry Institute (IQ-USP) in Brazil.
An article on a study led by Augusto published in the journal Chemical Research in Toxicology describes a novel method of detecting peroxymonocarbonate in cells based on the use of fluorescent molecular probes. This is the first time the substance has been detected in cells. The study was conducted under the aegis of the Center for Research on Redox Processes in Biomedicine (Redoxome), a Research, Innovation and Dissemination Center (RIDC) funded by FAPESP.
“This research is important both because it has produced a method to show that peroxymonocarbonate is produced under certain conditions, including cellular conditions, and because it has raised this as a topic for discussion, a boon considering the scant attention paid to CO2 in the redox field,” Augusto said.
Measuring fluorescence
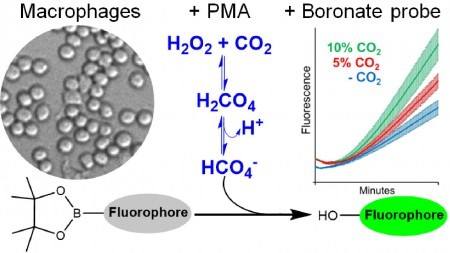
To detect peroxymonocarbonate, the researchers measured fluorescence from boronate probes. They first engineered an enzyme reaction that produced steady-state physiological concentrations of hydrogen peroxide and measured boronate probe fluorescence in the presence and absence of CO2. Boronates are used to detect hydrogen peroxide, peroxynitrite, hypochlorous acid and peroxymonocarbonate, among other substances, which react with them at different speeds and intensities, permitting the identification of these oxidants.
In the study, macrophages were activated to generate hydrogen peroxide. Macrophages are immune system cells that generate different oxidants, depending on the type of activation.
The researchers conducted several control experiments and concluded that the cells did not generate peroxynitrite or hypochlorous acid, but did generate peroxymonocarbonate in the presence of CO2.
“This is a relatively simple method for detecting peroxymonocarbonate in physiological concentrations of hydrogen peroxide and CO2. It used to be impossible, but researchers can now conclude that some effects observed in cells, such as higher oxidation of certain proteins or cell responses, may be due to peroxymonocarbonate, and they will be able to test this finding,” Augusto said.
Although peroxymonocarbonate has been known to chemists since the 1960s and has technological applications as a disinfectant and whitener, for example, its presence in cells was considered impossible owing to low concentrations of its precursors and a slow formation rate. According to Augusto, investigation of peroxymonocarbonate in biological systems began in the first decade of the millennium, initially with a focus on oxidative damage.
Redox signaling and CO2
Redox signaling is an adaptive response. “When stress increases slightly, the cell adapts. Formation of oxidants, for example, can lead to the expression of genes for antioxidant enzymes, responding to oxidative stress in this case. Moreover, many pathways that lead to cellular responses involve thiol proteins, which peroxymonocarbonate oxidizes faster than hydrogen peroxide,” Augusto said, adding that irreversible cellular damage occurs only when oxidant formation is very substantial.
Carbon dioxide is a precursor to peroxymonocarbonate, as is hydrogen peroxide. CO2 is naturally present in the atmosphere and in the human body. People exhale about 1.0 kg of CO2 per day on average as a product of their metabolism.
From a redox point of view, CO2 modulates the reactivity of hydrogen peroxide and peroxynitrite, two important metabolites of molecular oxygen. It also alters gene expression, including expression of genes involved in inflammation. And it is involved in protein nitration via peroxynitrite and in protein carbamylation, another post-translational modification that can alter the biological function of proteins.
Although more evidence of its role as a biological oxidant is needed, peroxymonocarbonate appears to be one of the possible intermediaries of the harmful effects of increased levels of CO2 in our bodies. Moreover, CO2 also acts through non-redox mechanisms, Augusto noted.
The article “Production of peroxymonocarbonate by steady-state micromolar H2O2 and activated macrophages in the presence of CO2/HCO3 – evidenced by boronate probes” is at: https://pubs.acs.org/doi/10.1021/acs.chemrestox.4c00059.
*With information from RIDC Redoxome.
Source: https://agencia.fapesp.br/52784