

A system developed by Brazilian researchers detects the presence of the virus in a nasopharyngeal swab in less than a minute.
Published on 01/24/2022
By Elton Alisson | Agência FAPESP – Researchers at São Francisco University (USF) in Bragança Paulista (São Paulo State, Brazil) have developed a technology that detects SARS-CoV-2 in under a minute directly from a nasal swab. The system can be used to diagnose COVID-19.
The development of the system was supported by FAPESP. The researchers at USF collaborated with colleagues at Mackenzie Presbyterian University (UPM), also in Brazil, and the University of Texas at Austin (UT Austin) in the United States. The project is reported in an article published in Analytical Chemistry.
“The new method permits direct analysis of swabs and produces a result in 45 seconds, facilitating the rapid screening of patients for COVID-19,” said Andréia de Melo Porcari, a professor at USF e last author of the article.
Collection of biological molecules
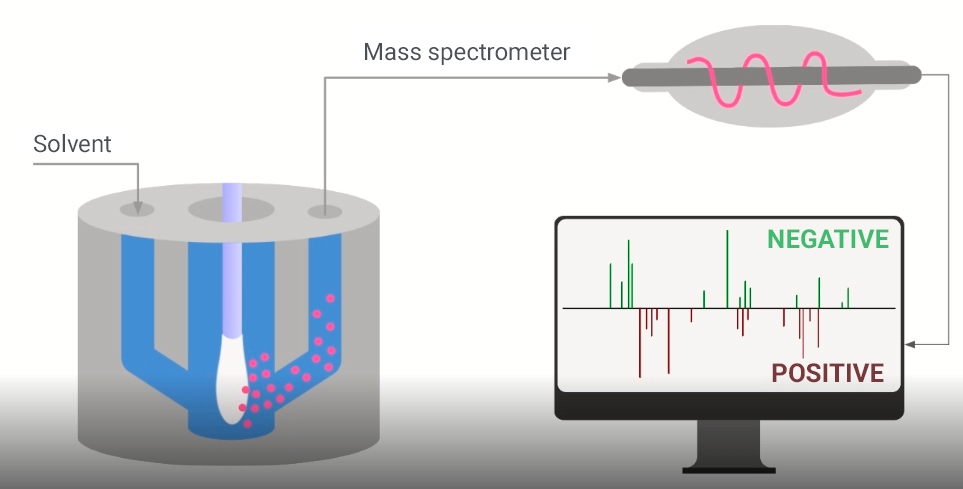
The technology is called MasSpec Pen and is derived from cancer detection and diagnostic system developed by Lívia Eberlin, a Brazilian researcher affiliated with UT Austin. It consists of a handheld device connected to a mass spectrometer, which identifies the chemical composition of substances in biological samples by their molecular mass and charge.
The MasSpec Pen, a sterilizable handheld plastic device, collects molecules from the surface of a tissue sample via water used as a solvent. The molecules are pumped through tubes to the mass spectrometer, where they are analyzed using machine learning algorithms and statistical models to see if the sample contains the cells of interest.
“The pen merely needs to touch the tissue for the water contained in the tip to extract the molecules to be analyzed,” Eberlin said.
In early clinical trials, the system distinguished between various types of cancer, including cells from thyroid, breast, lung, and ovarian tumors, and normal tissue with 96.3% overall accuracy. “The system will help pathologists and surgeons identify cancerous tissue more quickly and make more reliable decisions regarding treatment,” Eberlin said.
Detecting SARS-CoV-2
With the advent of COVID-19, the researchers decided to adapt the technology to detect SARS-CoV-2 directly from nasopharyngeal swabs. This entailed redesigning the pen and developing different solvents.
Because the pen touches only a tiny portion of the surface of a tissue sample, and the material collected by the swab is dispersed, the researchers inverted the pen so that the entire swab could be inserted into a chamber where it would come into contact with a small amount of chloroform-methanol, used as a solvent to extract the molecules from the nasopharyngeal secretion.
The molecules are sucked through a hole in the chamber to the mass spectrometer, which analyzes them to identify lipids that serve as viral markers. “The whole procedure takes less than a minute, including analysis. It’s a very fast cycle, with extremely short operational stages and no need to use any special reagents,” Porcari explained.
Validation of the technology
To validate the technology and methodology, the researchers analyzed nasopharyngeal swabs from 244 patients treated at Hospital Bragantino and Santa Casa Bragança Paulista at the start of the COVID-19 pandemic. The analysis identified the patients’ lipid profiles and served as a basis for the creation of statistical classifiers to distinguish positive symptomatic subjects from negative symptomatic and negative asymptomatic subjects.
The results of the study showed that the lipid profiles detected directly in the swabs using the new method permitted rapid screening of COVID-19 patients. “This new method of swab analysis can be adapted to detect many other viral and bacterial infections, and to conduct tests such as the cervical smear [or Pap smear, for prevention of cervical cancer],” Eberlin said.
The researchers now plan to obtain interlaboratory validation in partnership with UPM. “We want to demonstrate with independent samples that this new method of analysis is valid, regardless of the lab, equipment, and analyst doing the test,” Porcari said.
The article “Rapid screening of COVID-19 directly from clinical nasopharyngeal swabs using the MasSpec Pen” (doi: 10.1021/acs.analchem.1c01937) by Kyana Y. Garza, Álex A. Rosini Silva, Jonas R. Rosa, Michael F. Keating, Sydney C. Povilaitis, Meredith Spradlin, Pedro H. Godoy Sanches, Alexandre Varão Moura, Junier Marrero Gutierrez, John Q. Lin, Jialing Zhang, Rachel J. DeHoog, Alena Bensussan, Sunil Badal, Danilo Cardoso de Oliveira, Pedro Henrique Dias Garcia, Lisamara Dias de Oliveira Negrini, Marcia A. Antonio, Thiago C. Canevari, Marcos N. Eberlin, Robert Tibshirani, Livia S. Eberlin and Andreia M. Porcari is at: https://pubs.acs.org/doi/10.1021/acs.analchem.1c01937?ref=pdf&.
Source: https://agencia.fapesp.br/37795