

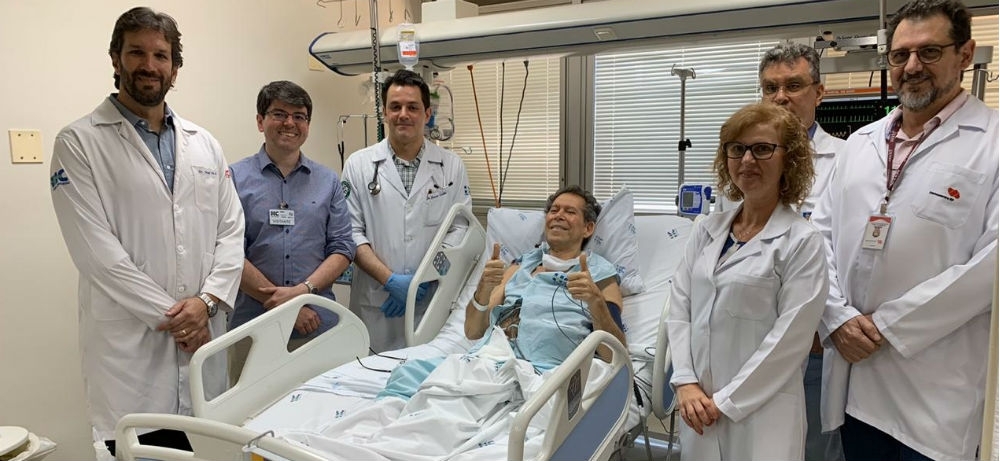
Patient with advanced refractory lymphoma was treated in Brazil by researchers at a center funded by FAPESP. The technique known as CAR T-cell therapy was used for first time ever in Latin America (photo: FMRP-USP)
Published on 05/12/2021
By Karina Toledo | Agência FAPESP – An innovative technique to treat cancer using reprogrammed cells from the patient has been tested for the first time in Latin America by researchers at the Center for Cell-Based Therapy (CTC) in Ribeirão Preto, São Paulo State.
CTC is a Research, Innovation and Dissemination Center (RIDC) funded by FAPESP and hosted by the University of São Paulo (USP).
Chimeric antigen receptor (CAR) T-cell therapy was used to treat an advanced case of diffuse large B-cell lymphoma (DLBCL), a common type of non-Hodgkin lymphoma. B-cells are white blood cells that fight infection. DLBCL develops when the body makes abnormal B-cells called lymphoma cells.
The 63-year-old patient had unsuccessfully been submitted to several different kinds of chemotherapy since 2017.
“The patient was expected to live for less than a year. In Brazil, only palliative care normally remains for cases like these. However, less than a month after infusion of CAR T-cells we observed an evident clinical improvement, and the patient was even able to do without pain medication,” said Renato Cunha, a researcher affiliated with CTC. Cunha heads the Bone Marrow Transplant and Cell Therapy Program at the teaching and general hospital run by the University of São Paulo’s Ribeirão Preto Medical School (HC-FMRP-USP).
CAR T-cell therapy was originally developed in Israel. In the United States, the two therapies approved by the FDA so far cost about USD400,000. Hospitalization is an additional expense. CTC’s methodology costs approximately BRL150,000 (now about USD36,000) and may become cheaper once it is used on a large scale.
“The technology is very recent, and our success in this case puts Brazil on an equal footing with the developed countries. It’s an achievement of the utmost social and economic importance to the nation,” said Dimas Tadeu Covas, principal investigator of CTC and of the National Institute of Science and Technology in Stem Cells and Cell Therapy, supported by FAPESP and the National Council for Scientific and Technological Development (CNPq), a federal government funding agency.
The therapy was administered to the patient by CTC and the Blood Center at HC-FMRP-USP as compassionate treatment, in which an unapproved or investigational therapy may be used to help a patient suffering from a serious condition for which no other therapy is available. The group now plan to conduct a trial with a number of volunteers. “We already have two other patients with high-grade lymphomas in the process of receiving infusions of reprogrammed cells,” Cunha said.

How it works
The researchers take blood samples from the patients to be treated and isolate a type of white blood cell called T-lymphocytes, or simply T-cells. T-cells are of key importance to the immune system thanks to their capacity to recognize antigens on the surface of pathogen or tumor cells and trigger production of antibodies.
With the aid of a viral vector (a virus genetically modified in the lab), a new gene is introduced into the T-cell nucleus. As a result, the T-cell expresses on its surface a receptor (protein) that will recognize the specific antigen in the tumor to be combated.
“The receptor is called chimeric because it’s mixed. Part of a receptor that already exists in the T-cell is connected to a new receptor that’s part of an antibody that can recognize the antigen CD19 [anti-CD19]. With this modification, the T-cells are redirected to recognize and attack the tumor cells,” Cunha explained.
The reprogrammed T-cells are “expanded” in the lab (placed in a culture medium so that they proliferate) and then infused into the patient. Before the treatment, a moderate dose of chemotherapy is administered to prepare the patient’s organism.
“About 24 hours after the infusion of CAR T-cells, an inflammatory response begins, indicating that the modified lymphocytes are reproducing and triggering the release of pro-inflammatory substances to eliminate the tumor. In addition to fever, there may also be a sharp drop in blood pressure [inflammatory shock], requiring admission to an intensive care unit. The physician in charge must have experience with the technique and monitor the patient continuously,” Cunha said.
The retiree submitted to the protocol at HC-FMRP-USP on September 9, 2019, has surmounted the critical stage of the treatment and has stopped taking morphine, previously administered at the maximum dose. He no longer has swollen neck lymph nodes.
“Besides these clinical signs of improvement, we have detected CAR T-cells in his blood. This is the clearest proof that the methodology worked,” Cunha said.
According to Cunha, it will be necessary to wait about three months for an accurate assessment of the patient’s response to the therapy, especially to see if it is partial or total. This depends on the biological profile of the tumor. The reprogrammed T-cells can stay in the organism for the rest of the patient’s life but they may equally well disappear after a few years.

Brazilian version
The project that led to the production of CAR T-cells in Brazil began four and a half years ago when FAPESP renewed its support for CTC. This period saw fundamental research on the viral constructs most widely used for genetic modification and the establishment of animal models for preclinical studies. Some 20 researchers are involved in the project, including physicians, cellular and molecular biologists, and engineers specializing in large-scale cell culture.
More recently, Cunha joined the team with clinical and laboratory experience acquired during an internship at the National Cancer Institute, part of the US National Institutes of Health (NIH) and a pioneer of the technique. In July 2018, Cunha received a Global Research Award from the American Society of Hematology (ASH), through which his team will receive USD150,000 in funding to help develop the technique at the Ribeirão Preto Medical School. The project as a whole was funded by BNDES (the national development bank), the São Paulo State Department of Health, the Ministry of Health and FINEP, the Brazilian Innovation Agency, as well as FAPESP and CNPq.
“The methodology we’ve developed is specific for the treatment of lymphoma, but the same logic can be used for any type of cancer. We’re working on protocols for the treatment of acute myeloid leukemia and multiple myeloma. We’re also talking to a Japanese university about partnering on treatments for solid tumors such as pancreatic cancer,” said Rodrigo Calado, a professor at FMRP-USP and a member of CTC.
The group’s goal, according to Calado, is to develop affordable therapies for low- and middle-income countries. In Brazil the researchers want the treatment to be included in the list of procedures funded by SUS, the national health system.
“The cost of our approach to CAR T-cell therapy is very similar to the amount paid by SUS for a bone marrow transplant, currently about BRL110,000, so the treatment can be considered affordable,” Calado said.
For Covas, CTC has a long track record in groundbreaking therapies, including the use of mesenchymal stem cells to treat diabetes and bone marrow transplants for sickle cell anemia patients.
“We were able to develop our CAR T-cell therapy protocol relatively quickly because we’ve been building these foundations for a long time. The fruits of FAPESP’s investment in basic science, training and research infrastructure are now being reaped in more effective and novel treatments for cancer,” Covas said.
Source: https://agencia.fapesp.br/31675