

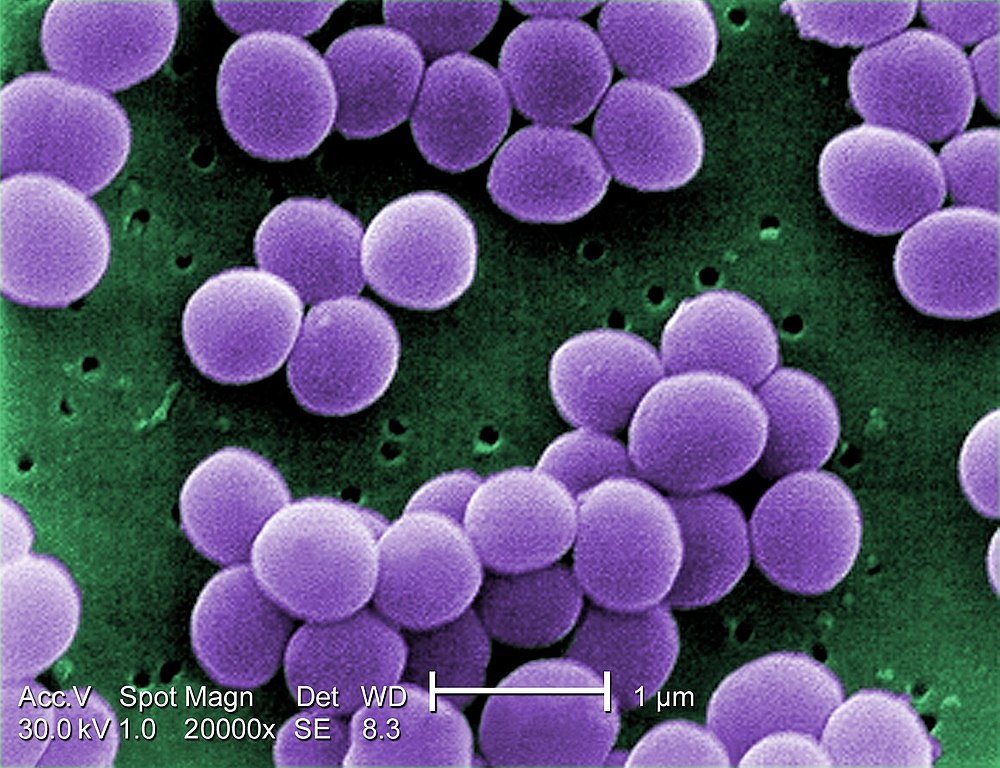
The research group analyzed patient samples containing Staphylococcus aureus, a bacterium that causes a range of diseases from skin infections to pneumonia (image Janice Haney Carr/CDC PHIL)
Published on 12/18/2023
By Luciana Constantino | Agência FAPESP – The development of antibiotics to combat multidrug-resistant bacteria, especially those that infect the airways, has been increasingly difficult, and some scientists have opted to try to weaken the bacteria so that the available therapeutic substances are made more effective.
This approach is promising, as shown by an article published in the journal Proceedings of the National Academy of Sciences (PNAS), which concludes that photodynamic inactivation (PDI) showed a novel capacity to modify bacterial sensitivity to antibiotics according to dosage, reducing the resistance and persistence of both standard and clinical strains.
The lead author is Vanderlei Salvador Bagnato, a physicist and materials engineer at the University of São Paulo’s São Carlos Institute of Physics (IFSC-USP) in Brazil.
The study focused on Staphylococcus aureus, a bacterium that causes a range of diseases from skin infections to pneumonia, investigating the effects of photodynamic action on resistant bacteria collected from patients and bacterial cells with laboratory-induced resistance. The results showed that five cycles of PDI were sufficient to break their resistance.
In PDI, a dye called a photosensitizer is energized by absorbing visible light to form reactive oxygen species that can oxidize and destroy microorganisms or weaken their resistance to antibiotics.
The researchers used 10 μM curcumin as the photosensitizer and worked with three antibiotics – amoxicillin, erythromycin and gentamicin. After the five cycles of PDI, they found that S. aureus was most susceptible to gentamicin, although the other two antibiotics also proved effective against the bacteria after PDI.
“We discovered that PDI doesn’t always destroy the bacteria, but it does destroy part of the mechanisms they use to become drug-resistant. This led to the idea of trying an oxidative shock to make them susceptible to antibiotics,” Bagnato told Agência FAPESP.
Bagnato is principal investigator for the Optics and Photonics Research Center (CePOF), one of the Research, Innovation and Dissemination Centers (RIDCs) supported by FAPESP, which also funded the study via two other projects (14/50857-8 and 19/12694-3).
The first author of the article is Jennifer Soares, a researcher at IFSC-USP and CePOF. As a former PhD candidate, she studied under Bagnato and co-author Kate Cristina Blanco, also a professor at IFSC-USP and a member of CePOF.
Multidrug-resistant bacteria
The World Health Organization (WHO) has prioritized antimicrobial resistance (AMR) as one of the top ten global public health threats facing humanity. AMR is a process that occurs as bacteria, viruses, fungi and parasites change over time and no longer respond to antibiotics and antivirals, for example.
The WHO estimates that some 1.2 million deaths are caused directly by AMR every year, and almost 5 million are indirectly associated with it. AMR could cost the global economy USD 100 trillion by 2050 if no action is taken.
According to a report issued last year by the WHO, out of every 100 patients in acute-care hospitals, seven patients in high-income countries and 15 patients in low- and middle-income countries will acquire at least one healthcare-associated infection (HAI) during their hospital stay. On average, 1 in every 10 affected patients will die from their HAI. “Deaths are increased two to threefold when infections are resistant to antimicrobials,” the report adds.
According to the PNAS article, the chances of approval of new antibiotics by the US Food and Drug Administration (FDA) for clinical trials in humans is 6 out of 10, and the probability that those approved will be a new antibiotic class is only 25%, which “implies a low probability of solving the bacterial resistance problem since most new antimicrobials tend to derive from existing classes”.
Bagnato’s research has focused for several years on drug-resistant pneumonia, one of the drug-resistant infections that most frequently cause deaths in intensive care units. “We’re about to publish an article describing a technique applied directly in the lungs. The patient inhales an inductive molecule, and we do extracorporeal infrared illumination to weaken the microorganism’s resistance as part of a strategy to combat pneumonia, for example,” he said.
Since February 2023, Bagnato has been working at Texas A&M University in the US, where he was invited to set up a biophotonics laboratory similar to CePOF on the main campus in College Station. He is currently on leave from IFSC-USP, but continues to collaborate on research projects in Brazil (read more at: revistapesquisa.fapesp.br/en/vanderlei-bagnato-this-isnt-a-case-of-brain-drain/).
The article “Recovering the susceptibility of antibiotic-resistant bacteria using photooxidative damage” is at: www.pnas.org/doi/10.1073/pnas.2311667120.
Source: https://agencia.fapesp.br/50500