

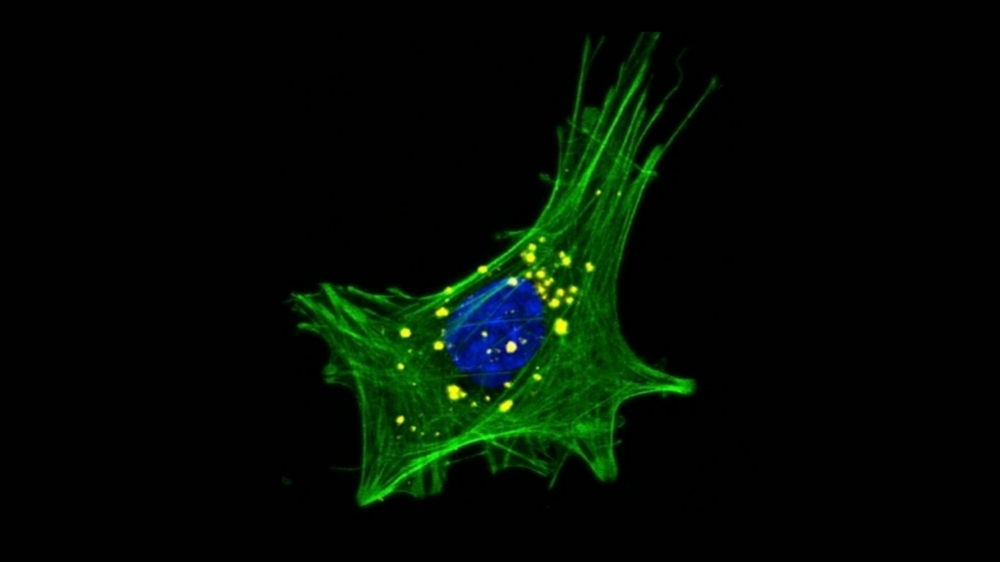
Alpha-synuclein aggregation in intestinal cells. This confocal microscope image shows an enteroendocrine cell, with the cell nucleus stained blue, actin cytoskeleton (green), and cytoplasm aggregates of alpha-synuclein (credit: Matheus de Castro Fonseca/CNPEM)
Published on 04/18/2022
By José Tadeu Arantes | Agência FAPESP – There is growing evidence that the gut microbiota, the collection of bacteria, viruses, fungi and other microorganisms that colonize the gastrointestinal tract, can influence the development and progression of neurodegenerative disorders. Two recently published articles by Brazilian researchers reinforce this hypothesis and describe a mechanism by which the development of Parkinson’s disease may be due in part to an imbalance between pathogenic and beneficial bacteria in the intestines known as dysbiosis.
The investigation was conducted with FAPESP’s support by researchers affiliated with the Brazilian Bioscience National Laboratory (LNBio) at the Brazilian Center for Research in Energy and Materials (CNPEM) in Campinas, São Paulo state. Some of the results were reported in February in an article published in the journal iScience. A second article appeared in March in the journal Scientific Reports.
“Research has shown that Parkinson’s is often diagnosed late and that it may originate much earlier in the enteric nervous system [which controls gastrointestinal motility], before advancing to the brain via autonomic fibers,” Matheus de Castro Fonseca, principal investigator for the study, told Agência FAPESP. Fonseca is currently doing postdoctoral research on the subject at the California Institute of Technology (Caltech) in the United States.
Recent publications have consistently reported the existence of gut dysbiosis in patients with sporadic Parkinson’s, as the non-inherited form of the disease is known, and shown that the bacteria Akkermansia muciniphila is abnormally abundant in fecal samples from these patients compared to controls.
“Specific cells in the gut epithelium, called enteroendocrine cells, have recently been found to have many neuron-like properties, including expression of the protein alpha-synuclein [αSyn]. Parkinson’s and other neurodegenerative diseases are known to be associated with abnormal accumulation and aggregation of this protein,” Fonseca said. “Because they’re in direct contact with the gut lumen – the space inside the intestines – and connected by synapses to the enteric neurons, enteroendocrine cells form a neural circuit between the gastrointestinal tracts and the enteric nervous system. As such, they may be a key factor in the emergence of Parkinson’s in the gut.”
With this knowledge in mind, the CNPEM group set out to see if substances secreted by A. muciniphila might trigger α-Syn aggregation in enteroendocrine cells and whether the αSyn aggregated in these cells could then migrate to peripheral nerve terminals in the enteric nervous system.
“We cultured proteins secreted by these bacteria in the absence of intestinal mucus and found them to lead to intracellular calcium overload in enteroendocrine cells, stressing their mitochondria [energy-producing organelles], triggering synthesis and release of reactive oxygen species [an excess of which damages intracellular structures], and causing αSyn aggregation,” Fonseca said. “Moreover, when we cultured enteroendocrine cells and neurons together, we found that aggregated αSyn can be transferred from one to the other.”
The discovery is very important because it shows that dysbiosis can boost the growth of bacteria that could contribute to αSyn aggregation in the gut, and that the protein may then migrate to the central nervous system, configuring a possible mechanism for the development of sporadic Parkinson’s.
“The cascade of reactions can start in the gut and move up into the brain,” Fonseca said. “People predisposed to sporadic Parkinson’s usually suffer from recurring constipation many years before they manifest the disease. In our study with animal models, we found a direct correlation between gut dysbiosis and Parkinson’s.”
New prevention strategies
Research on the microbiomes present in the human organism is advancing rapidly, as is scientists’ understanding of the links between an imbalance in gut microbiota and neurodegenerative disorders, from Parkinson’s and Alzheimer’s to autism. Dietary changes aimed at restoring a balance to the gut, as well as non-invasive transplantation of microbiota using capsules, can be important strategies to prevent these diseases.
“Neurodegenerative diseases are incurable right now, so prevention is fundamental,” Fonseca said. “Research used to focus on the brain, and little progress was made in this direction for decades. We’re now focusing on the gut instead. The latest discoveries seem highly promising. It’s much easier to modulate the gut microbiota than to deal with a well-established disorder in the central nervous system.”
The study was funded by FAPESP via a Regular Research Grant and a master’s scholarship. It also benefited from use of facilities and equipment at the National Institute of Photonics Applied to Cellular Biology, hosted by the University of Campinas (UNICAMP) and funded by FAPESP and the National Council for Scientific and Technological Development (CNPq).
Both studies by Fonseca’s group are open-access and can be read online.
The article “Transcellular propagation of fibrillar α-synuclein from enteroendocrine to neuronal cells requires cell-to-cell contact and is Rab35-dependent” is at: www.nature.com/articles/s41598-022-08076-5.
The article “Akkermansia muciniphila induces mitochondrial calcium overload and α-synuclein aggregation in an enteroendocrine cell line” is at: www.cell.com/iscience/fulltext/S2589-0042(22)00178-X?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS258900422200178X%3Fshowall%3Dtrue.
Source: https://agencia.fapesp.br/38424