

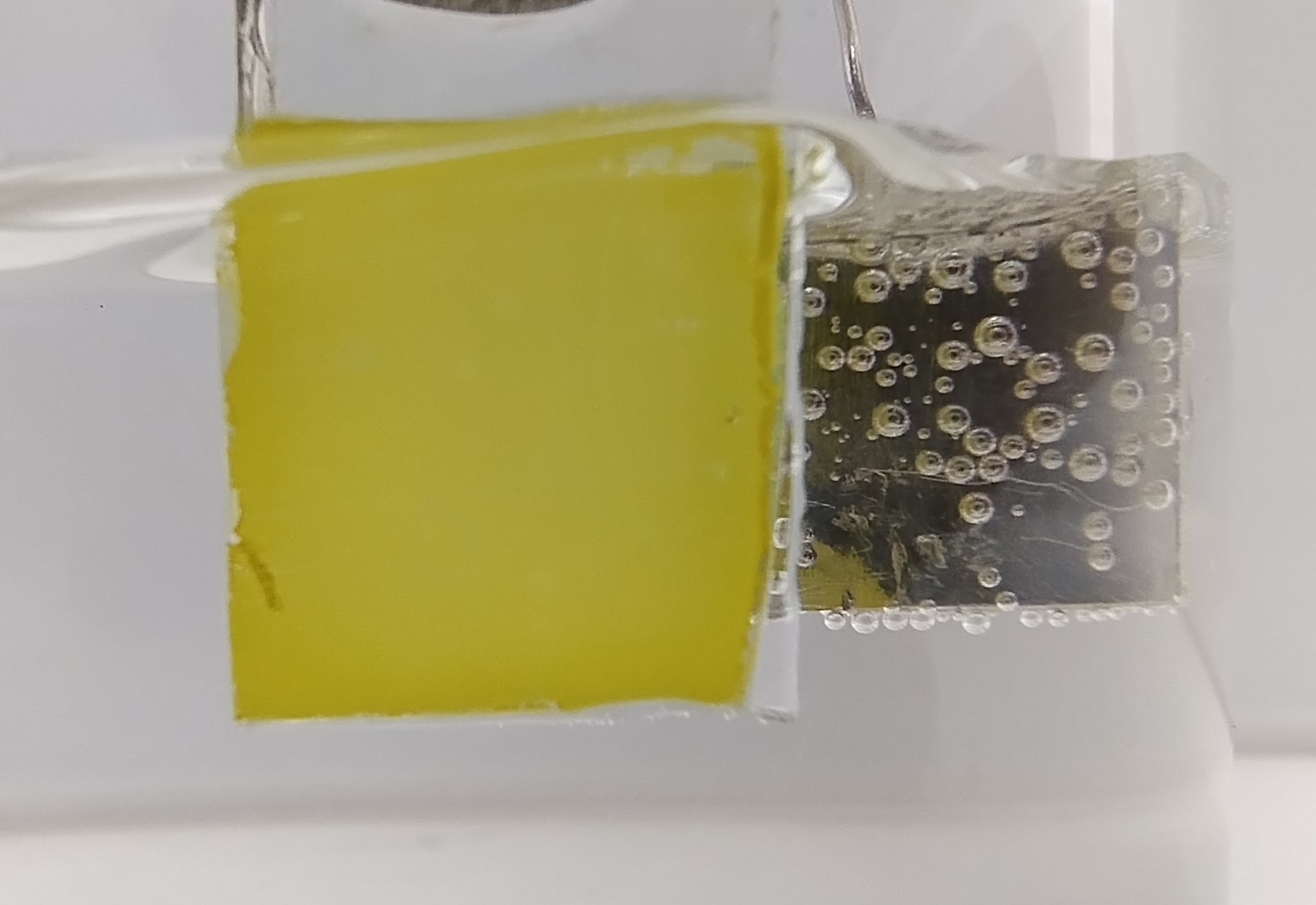
Glycerin oxidation takes place on the bismuth vanadate photoanode (yellow), while hydrogen (bubbles) is generated on the other electrode (image: CINE)
Published on 10/22/2025
Agência FAPESP* – A study led by researchers from the Center for Innovation in New Energies (CINE) has shown that replacing water with glycerol – popularly known as glycerin – increases the efficiency of photoelectrochemical cells. These cells use sunlight as a source of clean, renewable energy to generate green hydrogen.
CINE is an Applied Research Center (ARC) set up by FAPESP and Shell in 2018. It is based at the State University of Campinas (UNICAMP), the University of São Paulo (USP), and the Federal University of São Carlos (UFSCar), and eight other Brazilian institutions participate.
In photoelectrochemical cells, sensitive materials in the photoanode absorb light and drive a series of oxidation (loss of electrons) and reduction (gain of electrons) reactions, ultimately splitting the water molecule into oxygen and hydrogen molecules. However, water oxidation is slow and inefficient, which is why research groups around the world are looking for ways to overcome this limitation.
One approach is to replace water with molecules that can be more easily oxidized and generate larger electric currents, thereby making hydrogen production more efficient. The goal is to use organic molecules obtained from renewable, sustainable sources, such as biomass waste.
“The process of generating hydrogen requires energy, which can come from the electricity grid, as in classic electrolysis, or from other sources, such as solar energy,” explains Elton Sitta, a professor at UFSCar and a researcher at CINE. “In our study, we examined organic molecules that, when oxidized, can serve as sources of electrons and protons for generating hydrogen,” says Sitta, who led the study published in the journal Electrochimica Acta.
The authors compared the oxidation of methanol, ethylene glycol, and glycerol molecules on bismuth vanadate photoanodes. This material is considered promising for this type of application due to its good light absorption, low toxicity, low cost, and stability in the presence of humidity and light.
The research team concluded that glycerol exhibits the greatest potential for generating electrons for the production of green hydrogen. In addition, it is widely available in Brazil because it is a by-product of biodiesel production. Finally, in addition to electrons, its oxidation produces substances that can be used as raw materials in various industries.
“For large-scale production, there are challenges for both the oxidation of water and organic molecules as a source of electrons, but organic molecules have proved to be an interesting alternative to avoid the corrosion of photocatalysts and the possibility of obtaining other value-added products,” says Sitta.
The research, which was carried out at UNICAMP and UFSCar, was funded by FAPESP through four projects (13/07296-2, 17/11986-5, 23/02929-9, and 22/02300-0). The article is also signed by Cristian Hessel, Lauren Moreti, Victor Yoiti Yukuhiro, and Pablo S. Fernández.
The article “Methanol, ethylene glycol, and glycerol photoelectrochemical oxidation reactions on BiVO4: Zr,Mo/Pt thin films: A comparative study” can be read at: www.sciencedirect.com/science/article/abs/pii/S0013468624015366?via%3Dihub.
* With information from Verónica Savignano from CINE.
Source: https://agencia.fapesp.br/56248