

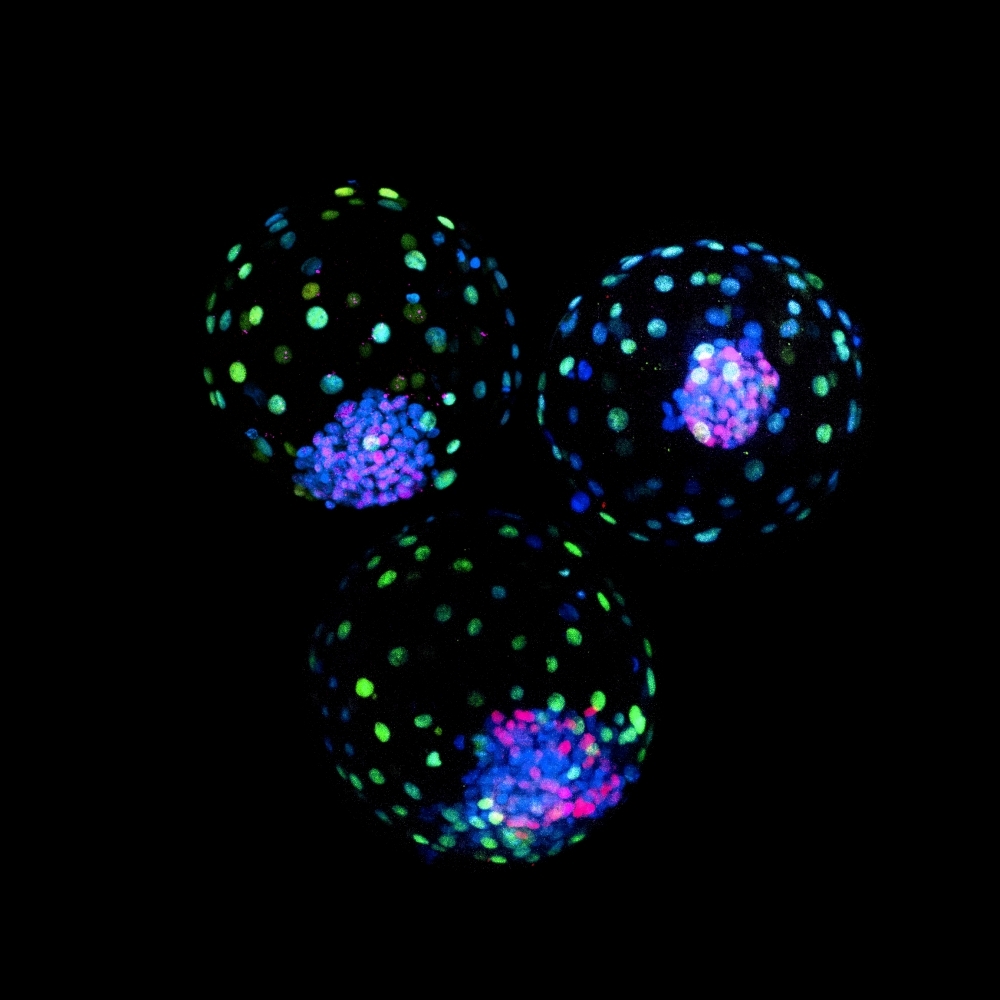
The results pave the way for breeding of cattle with selected traits. Preliminary work findings for human embryo models recently published by international groups could contribute to the understanding of congenital defects and early pregnancy loss (image: confocal microscope image of bovine blastoids assembled from trophoblast stem cells and expanded potential stem cells; credit: Carlos A. Pinzón Arteaga)
Published on 07/24/2023
By Ricardo Muniz | Agência FAPESP – An article published in the journal Cell Stem Cell describes the first scientific study to develop blastoids – “synthetic embryos”, as they were initially (and incorrectly) called – from bovine pluripotent stem cells (PSCs). The blastoids resemble blastocysts, structures formed in the early embryonic development of mammals. The researchers conclude that they can be used in the short term to model bovine embryonic development.
“Because stem cells are easy to manipulate, it’s possible to edit specific genes and study the effects of doing so,” said Ana Elisa Ribeiro Orsi, a researcher at the University of São Paulo’s Institute of Biosciences (IB-USP) and one of the authors of the article. “In the long run, we believe blastoids can be refined to the extent of making complete gestation possible. This would facilitate the large-scale breeding of cattle with desirable characteristics.”
Orsi did part of her master’s research in Southwestern Medical Center’s Department of Molecular Biology at the University of Texas (USA), with FAPESP’s support. Her thesis advisor is Lygia da Veiga Pereira, a professor in the Department of Genetics and Evolutionary Biology at IB-USP.
Unlike hematopoietic stem cells (HSCs), which give rise only to blood cells, and mesenchymal stem cells (MSCs), which differentiate only into bone, cartilage and fat cells, PSCs can become all kinds of cell in an adult individual.
In the study, PSCs were first isolated from blastocysts three, five and seven days after fertilization in mouse, human and bovine cells respectively. At this stage, PSCs have not yet begun differentiating into other cell types. “We extracted these cells from embryos and multiplied them in culture to generate billions of cells, which can be induced to differentiate into any tissue of interest in the lab,” said Pereira, who heads the National Embryonic Stem Cell Laboratory (LaNCE) at USP and also participates in the Center for Cell-Based Therapy (CTC), one of the Research, Innovation and Dissemination Centers (RIDCs) funded by FAPESP.
According to the article, understanding blastocyst formation and implantation is critical to improve farm animal reproduction but hampered by a limited supply of embryos, and bovine blastoids are an accessible in vitro model for the study of embryogenesis. “Upon further optimization, bovine blastoid technology could lead to the development of new artificial reproductive technologies for cattle breeding, which may enable a paradigm shift in livestock reproduction,” the authors conclude.
The article describes the protocol created and notes that the blastoids are similar to bovine embryos in morphology and characteristic gene expression. “My favorite part is that when we transferred the blastoids to cows, we detected interferon-tau in their blood. Interferon-tau is a hormone that indicates maternal recognition of pregnancy in bovines,” Orsi said.
Besides Orsi, the article is signed by 13 other researchers affiliated with the University of Texas, the University of Florida and Louisiana State University in the USA, and Shanghai Jiao Tong University and the Institute of Zoology of the Chinese Academy of Sciences in China.
“The most exciting part of this research for me is the capacity of PSCs to organize in vitro and resume embryo development,” said Pereira, who has a PhD in biomedical sciences from City University of New York (USA) and is a member of the Board of Directors of the International Society for Stem Cell Research (ISSCR).
LaNCE-USP has differentiated PSCs into heart cells, blood vessel cells and neurons. Since 2012, it has reprogrammed adult cells to become induced pluripotent stem cells (iPSCs).
Human embryo models
According to a press release issued by the ISSCR on June 27, 2023, “Recent work presented at the ISSCR 2023 Annual Meeting in Boston, USA, this month and additional research posted online as preprints [without peer review] shortly thereafter highlight the rapid pace of progress in the development of stem cell-based embryo models.” Considering this acceleration, the release contains basic information to “aid public understanding of this progress and assist the media in accurate reporting,” such as the fact that “integrated embryo models are neither synthetic nor embryos. While these models can replicate aspects of the early-stage development of human embryos, they cannot and will not develop to the equivalent of postnatal stage humans”.
Furthermore, it continues, “the ISSCR Guidelines prohibit the transfer of any embryo model to the uterus of a human or an animal”.
Use of these models, the statement stresses, “allows experimental modeling of the early stages of embryonic development” and can facilitate understanding of early pregnancy loss and placental failure. It can also “help researchers gain basic knowledge of the early developmental origins of congenital defects in the heart, nervous system, and other organs”.
Anyone interested in more information, it concludes, should “consider reviewing Toward Guidelines for Research on Human Embryo Models Formed from Stem Cells and Snapshot: Embryo models”.
The article “Bovine blastocyst-like structures derived from stem cell cultures” is at: www.sciencedirect.com/science/article/abs/pii/S1934590923001212?via%3Dihub.
Source: https://agencia.fapesp.br/41961