

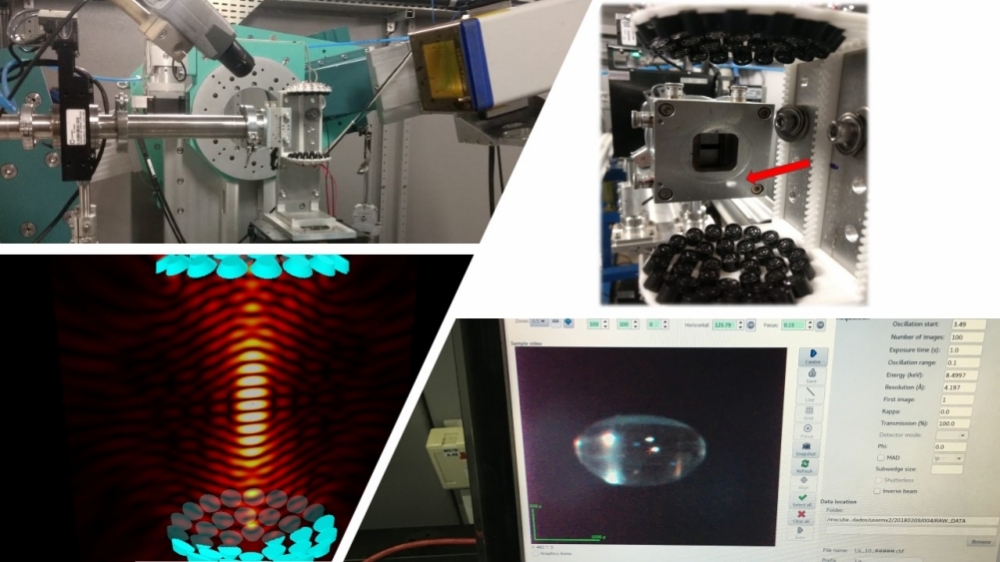
By combining acoustic levitation and X-ray diffraction with synchrotron light, the researchers were able to analyze the interactions of a drug’s atoms in real time and improve its formulation (photos: researchers’ archive)
Published on 04/11/2022
By Maria Fernanda Ziegler | Agência FAPESP – While droplets of a medication are levitating on ultrasonic waves, researchers are able to analyze the interactions of its atoms in real time with the aid of a powerful synchrotron light beam, and above all to check for the formation of crystals, which would spoil the formulation or make it less efficient for treatment of a disease.
This combination of techniques was tested by scientists affiliated with the University of São Paulo’s Physics Institute (IF-USP) and School of Pharmaceutical Sciences (FCF-USP) in Brazil on niclosamide, a medication for tapeworms that has also been found to combat tumors and viruses. The experiment was conducted at the Brazilian Center for Research in Energy and Materials (CNPEM) in Campinas, São Paulo state, where the Brazilian Synchrotron Light Laboratory (LNLS) is located. The research was supported by FAPESP via four projects (17/27078-0, 19/04998-2, 18/00273-0 and 18/11990-5).
“The pharmaceutical industry is investing heavily in a search for new technologies to develop medications. With this new approach, we succeeded in accelerating the development of more effective medications,” said Gabriel Araújo, a professor in the Pharmacy Department at FCF-USP.
Araújo explained that large amounts of the active ingredient are usually unavailable at the start of drug development, so that it is impossible to test a wide array of formulations and combinations of excipients (substances without direct therapeutic action, included to assure stability, among other reasons). When a drug is discovered, an issue to be resolved in parallel with large-scale chemical synthesis of the raw material, as well as animal and clinical trials, relates to optimizing the formulation and enhancing bioavailability. For a drug to be safe and perform effectively in the treatment of a specific disease, its formulation must have physical and chemical properties that enable the active ingredient to dissolve in the gastrointestinal tract, enter the bloodstream, and reach the targeted organ, for example.
Improvement of the formulation also permits repurposing of the drug for the treatment of other diseases, as in the case of niclosamide, an anti-parasite drug that has been on the market for decades but is administered orally with very low bioavailability.
“The scientific community has taken an interest in this drug because it has several other applications,” Araújo said. “Recent studies showed anti-tumor activity and potential for treatment of pulmonary arterial hypertension and viral infections. The studies were pre-clinical and new formulations will be needed if it’s to be effective in these applications.”
An article on the study is published in the International Journal of Pharmaceutics. It also reports a good match between the results of the new approach and those of conventional methods. “Levitation and X-ray diffraction with synchrotron light aren’t easy techniques to use, but the approach offers several advantages,” Araújo said. “We used a sample with only 20 microliters of the formulation. Conventional methods would have required a much larger sample. In addition, they’re more complex, involve several stages of sample preparation, and take much longer to analyze.”
Other advantages Araújo stressed are high sensitivity and the absence of interaction with surfaces during the analysis. “Levitation doesn’t involve any surfaces that come into contact with the sample. We know that surfaces can induce crystallization, as is common in conventional testing methods,” he said. “The technique is also very fast, thanks to the use of synchrotron light. Analysis takes only a few minutes, and sensitivity is high enough to detect recrystallization even below 1%.”
Using these two techniques, the researchers set out to understand how atoms interact in the drug, and also how the polymers used to enhance stability interact. In the case of niclosamide, the analysis was especially complex because the proposed formulation belonged to a class known as amorphous solid dispersions, unstable systems formed by dissolving crystals and dispersing them in polymers to increase solubility so that they are more efficiently absorbed when the drug is administered orally.
Araújo explained that the use of amorphous solid dispersions makes drugs more bioavailable but less stable, and recrystallization may occur while they are in storage, impairing their efficacy. “Amorphous dispersion is a widely used technology in the pharmaceutical industry,” he said. “It’s present in many medications that are now on the market. There are many drugs that don’t dissolve well in the gastrointestinal tract. It enhances the bioavailability of orally administered drugs in both pre-clinical and clinical trials.”
The same group of researchers has conducted studies to improve drugs that have been on the market for years and can be repurposed for other therapeutic applications via potentiation (to increase absorption and hence bioavailability, for example).
In another study, also reported in the International Journal of Pharmaceutics, the group used synchrotron light at Argonne National Laboratory, near Chicago in the United States, to analyze flubendazole, an anti-parasitic developed in the 1970s. The study was conducted in partnership with Purdue University in the US and was also supported by FAPESP.
“Recent research has shown that flubendazole has the potential for use in adjuvant cancer therapy, for example. However, its solubility is low, and currently this prevents its use to treat cancer because it doesn’t dissolve in the gastrointestinal tract. We set out to improve the formulation so that it can be administered orally,” Araújo said.
Although they did not use acoustic levitation in that study, they did analyze amorphous solid dispersions. “We found a link between the structure of amorphous dispersion and stability. We studied the interactions between flubendazole and a polymer called hydroxypropyl methylcellulose [a widely used tablet excipient]. This enabled us to predict where crystal formation occurs and plan the best formulation,” he explained.
The article “Acoustic levitation and high-resolution synchrotron X-ray powder diffraction: A fast screening approach of niclosamide amorphous solid dispersions” is at: www.sciencedirect.com/science/article/abs/pii/S0378517321004166#!.
The article “Amorphous dispersions of flubendazole in hydroxypropyl methylcellulose: Formulation stability assisted by pair distribution function analysis” is at: www.sciencedirect.com/science/article/abs/pii/S0378517321003057.
Source: https://agencia.fapesp.br/38370