

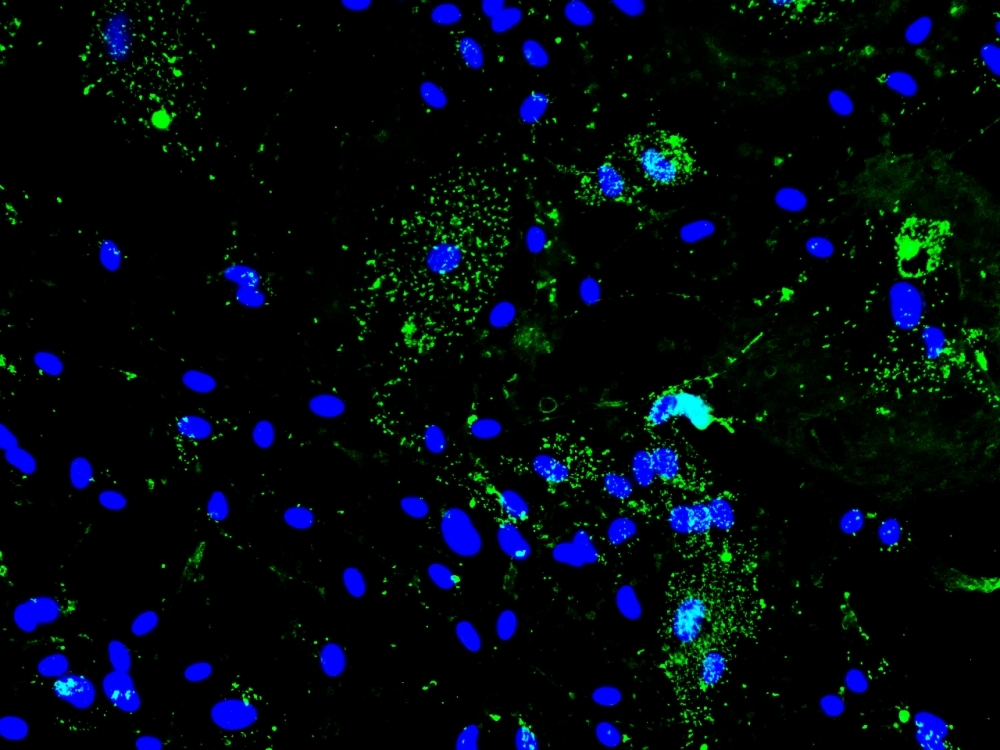
Experiments with hamsters and cultured astrocytes point to possible causes of neurological complications in COVID-19 survivors (SARS-CoV-2 spike protein (green) and astrocyte nuclei (blue); image: Lilian Gomes de Oliveira and Yan de Souza Angelo/ICB-USP)
Published on 11/22/2021
By Karina Toledo | Agência FAPESP – Impaired recent memory and mental confusion are among the most common neurological complications found in COVID-19 survivors, and experiments with hamsters conducted at the University of São Paulo (USP) in Brazil may help scientists understand how they come about, as well as pointing to possible ways of combating them.
The study involved both live animals and astrocytes isolated from the central nervous system of the rodents and cultured in vitro. The results suggest that infection by SARS-CoV-2 accelerates the metabolism of these nerve cells and increases consumption of molecules used as fuels for energy, such as glucose and the amino acid glutamine.
The main problem is that glutamine is also important to the synthesis of glutamate, the key neurotransmitter involved in communication between neurons, and this process is apparently impaired. In the study, the researchers observed the presence of the virus and alterations in levels of proteins associated with the energy metabolism in the hippocampus, the brain region essential to consolidation of memory and learning, and in the cortex, also important for memory, cognition, and language.
“SARS-CoV-2 appears to overactivate astrocyte metabolism to obtain more energy for replication of its genetic material and production of new viral particles. When we used a drug to block glutaminolysis [production of energy from glutamine], viral replication in the cultured cells was reduced by about a third,” said Jean Pierre Peron, principal investigator for the study. Peron is a professor in USP’s Biomedical Sciences Institute (ICB-USP), and a member of the Scientific Platform Pasteur-USP (SPPU), part of the Institut Pasteur International Network.
Researchers affiliated with other institutions who collaborated with Peron and his group included teams at the University of Campinas (UNICAMP) and the Center for Research on Inflammatory Diseases (CRID), a Research, Innovation and Dissemination Center (RIDC) supported by FAPESP and hosted by the Ribeirão Preto Medical School (FMRP-USP). The study was funded by FAPESP via seven projects (20/06145-4, 20/07251-2, 17/27131-9, 15/15626-8, 20/04579-7, 20/04746-0, and 15/25364-0). Preliminary results are described in a preprint posted to bioRxiv, as yet without peer review.
Previous evidence
Astrocytes are the most abundant central nervous system cells, and their roles include supporting the functioning of neurons by supplying nutrients such as glucose and glutamine. They also regulate the levels of neurotransmitters and other substances that may affect neuronal functioning, such as potassium, and are part of the blood-brain barrier, which protects the brain against pathogens and toxins.
In 2020, a group led by Thiago Cunha at FMRP-USP analyzed brain tissue samples collected from people who died of COVID-19 and confirmed the presence of SARS-CoV-2 inside astrocytes.
At UNICAMP, Daniel Martins-de-Souza and his group showed that the virus can infect and replicate in human astrocytes. In this study, the astrocytes were derived from induced pluripotent stem cells (iPSCs) using a method that consists of reprogramming adult cells from skin or other easily accessible tissues (more at: agencia.fapesp.br/34404).
Tests performed in the laboratory at the time showed that infection induced alterations in biochemical pathways associated with the energy metabolism. This finding has now been reinforced by the experiments conducted by the SPPU researchers.
“This entire set of data suggests that astrocytes and the cellular energy metabolism play a key role in impairment of the central nervous system in subjects infected with SARS-CoV-2,” Martins-de-Souza told Agência FAPESP.
Recent results
After infecting hamster astrocytes with SARS-CoV-2, the researchers found that the cells began producing inflammatory molecules (cytokines) and observed a change in the expression of proteins associated with the carbon metabolism (glucose). An analysis of the metabolites present in the cell culture found some substances to be significantly reduced compared with controls (uninfected astrocytes).
“The amount of glutamine and other molecules involved in producing energy and synthesizing proteins, such as aspartate, pyruvate and alpha-ketoglutarate, was smaller. This finding suggests the cells were strongly activated metabolically. We believe this was because the virus requires more energy to replicate,” Peron said.
In another experiment, cultured astrocytes were placed in a respirometer to measure glucose and oxygen consumption, and acceleration of the infected cells’ metabolism was confirmed.
“These are central nervous system cells, and the fact that levels of glutamine were reduced was significant because glutamine is the raw material for synthesis of glutamate and about 90% of our synapses are mediated by this neurotransmitter,” Person said. “Infection apparently causes an energy imbalance, which in turn leads to an imbalance in glutamate levels. This may alter the functioning of neurons, but further investigation is needed.”
When infected astrocytes were treated with a drug to block glutaminolysis, viral replication was reduced, in terms of a drop in both viral RNA levels and the quantity of viral particles in the culture medium.
In the in vivo experiments, hamsters were infected intranasally, and the presence of the virus in the central nervous system was monitored for 14 days. As had occurred in vitro, the infection induced production of inflammatory cytokines and also caused alterations in the brain protein profile.
“We observed the presence of viral particles in the hippocampus and cortex, both of which regions are rich in glutamate,” Person said. “We also observed alterations in several proteins associated with the carbon and glutamine metabolism. This suggests something similar may be occurring in humans and could be the origin of symptoms such as memory loss, cognitive impairment, difficulty concentrating, and mental confusion.”
According to Martins-de-Souza, reduced levels of glutamine had already been observed in tests with human astrocytes. “The new findings confirm that glutaminolysis is an important process for viral replication,” he said. “So we’re talking about a target in the brain for exploration in the search for therapies.”
For Peron, it will be most feasible in the near term to test treatment of neurological complications following COVID-19 with drugs that modulate glutamate-mediated synapses. This kind of drug is already used to treat Alzheimer’s disease.
The article “SARS-CoV-2 infection impacts carbon metabolism and depends on glutamine for replication in Syrian hamster astrocytes” is at: www.biorxiv.org/content/10.1101/2021.10.23.465567v1.
Source: https://agencia.fapesp.br/37383