

A technique created by researchers in Brazil and Spain prevents blocking of the anti-aging effect of vitamins C and B3 due to contact with air and sunlight (image: Leonardo Delello Di Filippo/UNESP)
Published on 12/12/2022
By Thais Szegö | Agência FAPESP – Researchers at São Paulo State University (UNESP) in Brazil and the University of Valencia in Spain have developed microparticles containing antioxidants that combat premature skin aging and remain stable in contact with oxygen and sunlight. The system is designed to assure sustained release of the drugs into the skin, prolonging their action.
In an article on the study published in the journal Life, the researchers describe how they encapsulated microparticles containing ascorbic acid (vitamin C) and nicotinamide (vitamin B3), which are known to protect the skin against damage from pollution, ultraviolet radiation and free radicals. Both vitamins stimulate collagen synthesis and act as depigmenting agents, eliminating dark skin spots. In addition, vitamin B3 combats acne and rosacea by reducing inflammatory processes and production of skin grease.
However, both vitamins are unstable and degrade easily owing to changes in pH and temperature, as well as exposure to sunlight, water and oxygen, making their use in cosmetics a challenge that the researchers set out to address.
The study was funded by FAPESP via five projects (15/19097-0, 16/20957-6, 15/02619-3, 20/16573-3 and 19/25125-7).
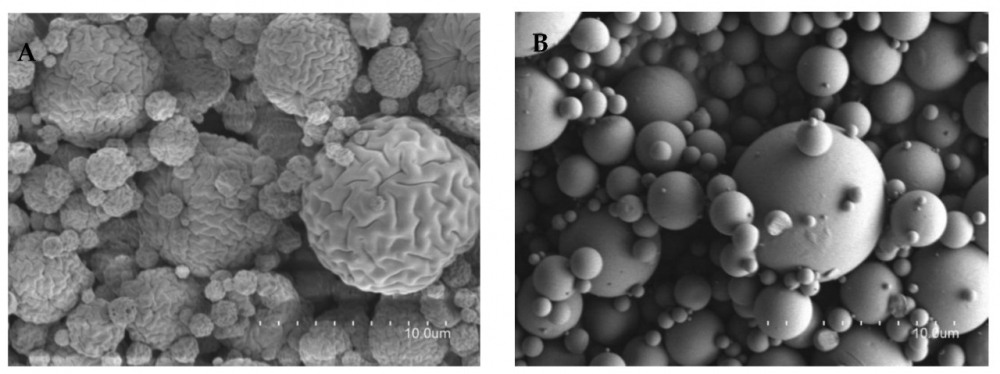
The researchers encapsulated the two vitamins in microparticles of chitosan, a compound derived from the shells of crustaceans, to increase their stability, prevent premature oxidation and provide antimicrobial protection, which enhances cosmetics for skin treatment.
“We added ethanol as a solvent to mix the substances in the microparticles. We then used a spray dryer under controlled temperature and pressure to dry the solvent, leaving the bioactive particles in the form of an orange-colored powder,” said Leonardo Delello Di Filippo, first author of the article and a PhD candidate at UNESP’s Araraquara School of Pharmaceutical Sciences (FCFAr).
During the procedure, they added other materials to give the particles a smooth and uniform surface, which is a desirable feature of controlled release systems, and increase the bioavailability of the drugs in the skin.
“An analogy with a car journey may help. If the road is full of potholes, accidents are more likely. If the road is nice and smooth, like the surface of the microparticles, everything works better,” Di Filippo said.
Preclinical trials
The researchers conducted preclinical trials using in vitro laboratory models and skin from pig ears, with cells, structure and thickness closely resembling those of human skin. The two processes were complementary and proved that the microparticles prolonged the action of the drugs in the skin thanks to controlled release, had antibacterial properties, helped protect tissue layers against contamination, and assisted their own conservation.
In addition to all the benefits for skin product formulation, the technique used to produce the microparticles is easily reproducible and industrially scalable. “It’s a value-added multiuse product with a distinctive technology. For all these reasons, its use by the cosmetics industry is increasingly plausible,” Di Filippo said.
According to Marcos Antonio Corrêa, a professor at FCFAr-UNESP’s Department of Drugs and Medications and Di Filippo’s thesis advisor, production of the microparticles on an industrial scale would enable cosmetics companies to make use of the technology. “The researchers have been contacted by an interested company, and negotiations are in progress,” he said.
The article “In vitro skin co-delivery and antibacterial properties of chitosan-based microparticles containing ascorbic acid and nicotinamide” is at: www.mdpi.com/2075-1729/12/7/1049/htm.
Source: https://agencia.fapesp.br/40285