

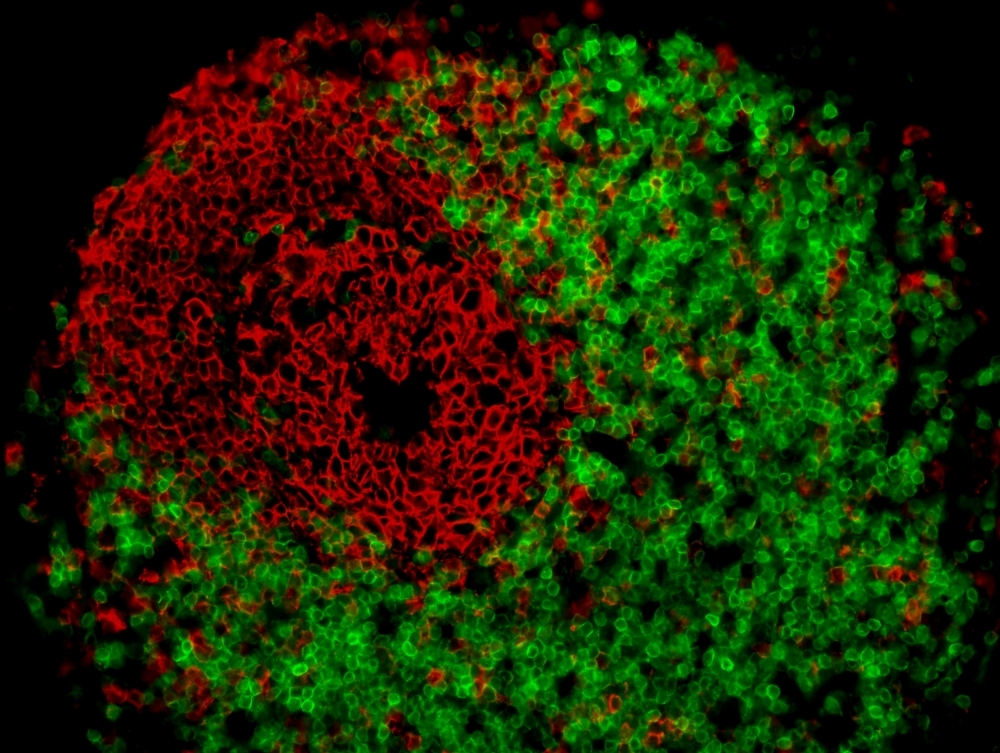
Analysis of tissue samples showed the presence of cells that can trigger the inflammatory process typical of Crohn’s. The study paves the way for the detection of biomarkers that can help predict evolution to the severe form of the disease, and for more precise diagnosis (lymphoid follicle in mesenteric adipose tissue from Crohn’s patient, illustrating presence of positive CD20 and CD3 cells; image: researchers’ archive)
Published on 11/01/2021
By André Julião | Agência FAPESP – A group of researchers affiliated with institutions in Campinas, Brazil, and Barcelona, Spain, has identified a set of genes whose activity is altered in the intestine of patients with Crohn’s disease, a disease that causes inflammation of the digestive tract and can lead to abdominal pain, severe diarrhea, weight loss and malnutrition. The discovery enriches scientists’ understanding of the disease and could help medical teams develop more effective prognostic methods and treatments. The study was part of a research project funded by FAPESP.
A paper reporting the findings is published in the Journal of Translational Medicine. The authors are researchers at the University of Campinas (UNICAMP) in the state of São Paulo and Barcelona Clinic Hospital in Spain.
Described in 1932 by Burrill B. Crohn (1884-1983), the disease is linked to an imbalance in the immune system, and its origins are both genetic and environmental. Among its features is an increase in mesenteric adipose tissue, a layer of fat between the gut and liver, involving inflammation of the intestinal folds. This leads to diarrhea in mild cases, and bowel perforation or obstruction requiring surgery in severe cases.
“We don’t know whether the disease is caused by thickening mesenteric adipose tissue or produces it, but our study showed that this tissue contains memory immune cells. These may trigger a recurrence of the disease in response to some stimulus, or exacerbate the inflammatory process,” said Raquel Franco Leal, a professor at UNICAMP’s School of Medical Sciences (FCM) and principal investigator for the study.
The researchers sequenced RNA from samples of mesenteric adipose tissue and the ileum (the final section of the small intestine) supplied by two groups of patients. One underwent surgery for Crohn’s disease, while the control group underwent surgery for other conditions not linked to inflammatory disorders.
“Sequencing enabled us to find out which immune pathways were most activated by the disease,” Leal said. “We concluded that its main signature is the presence of plasma cells, a type of defense cell that secretes antibodies.”
The lead authors of the paper are Francesca Aparecida Ramos da Silva, who conducted the study as part of her PhD research at FCM-UNICAMP, with a scholarship from CAPES, the Brazilian Ministry of Education’s Coordination for the Improvement of Higher Education Personnel, and Lívia Bitencourt Pascoal, who completed a postdoctoral fellowship at the institution, also with a scholarship from CAPES.
The study won two prizes at the 67th Brazilian Coloproctology Conference (CBP), held in the second half of 2019. One was the Angelita Habr-Gama Prize, created in honor of the first woman to become a full professor of surgery at the University of São Paulo’s Medical School (FM-USP).
Genes involved
Sequencing of RNA from the mesenteric adipose tissue samples pinpointed 17 genes that were overexpressed or underexpressed compared with people without inflammatory bowel disease. In particular, the researchers noted overexpression of genes associated with plasma cell activity.
The ileum samples yielded 849 genes expressed more or less than in healthy individuals. In the two sets of samples, a total of 204 genes were found to be significantly altered when compared with unaffected tissue.
“The study brought to light the role played by mesenteric adipose tissue in storing immune cells and also the involvement of immune responses controlled by antigens, indicated by the presence of plasma cells,” said Leal, who heads UNICAMP’s Inflammatory Bowel Disease Research Laboratory (LABDII).
Unlike rheumatoid arthritis, lupus and psoriasis, for example, Crohn’s disease is not considered an autoimmune disorder because no circulating antibodies to the disease have been identified that attack any of the body’s structures or cells. Nevertheless, the study emphasizes the key role played by the immune system in Crohn’s, which is classified as immune-mediated.
The researchers plan to continue monitoring the patients whose mesenteric adipose tissue they analyzed, to see how progression of the disease in the years ahead correlates with the set of altered genes in each one. The hope is that in the future it may be possible to predict post-operative progression or relapse.
“Crohn’s can be hard to manage because the symptoms and clinical evolution vary a great deal from one patient to another. We want to understand the severe forms of the disease, in which intestinal fold inflammation can lead to fistulas [connections to adjacent organs], abscesses, spontaneous perforation or bowel obstruction, requiring surgery,” Leal said.
In addition, it could become feasible to prescribe treatments that are better targeted to patient profiles. Because Crohn’s is an immune-mediated disease, many patients take medication that reduces immune system activity, which is itself a problem during the COVID-19 pandemic.
“We’re asking our patients to go to hospital only in cases of extreme necessity. They’re in the coronavirus high-risk group,” Leal said.
The article “Whole transcriptional analysis identifies markers of B, T and plasma cell signaling pathways in the mesenteric adipose tissue associated with Crohn’s disease” (doi: 10.1186/s12967-020-02220-3) by Francesca Aparecida Ramos da Silva, Lívia Bitencourt Pascoal, Isabella Dotti, Maria de Lourdes Setsuko Ayrizono, Daniel Aguilar, Bruno Lima Rodrigues, Montserrat Arroyes, Elena Ferrer-Picon, Marciane Milanski, Lício Augusto Velloso, João José Fagundes, Azucena Salas and Raquel Franco Leal is at: translational-medicine.biomedcentral.com/articles/10.1186/s12967-020-02220-3.
Source: https://agencia.fapesp.br/37214