

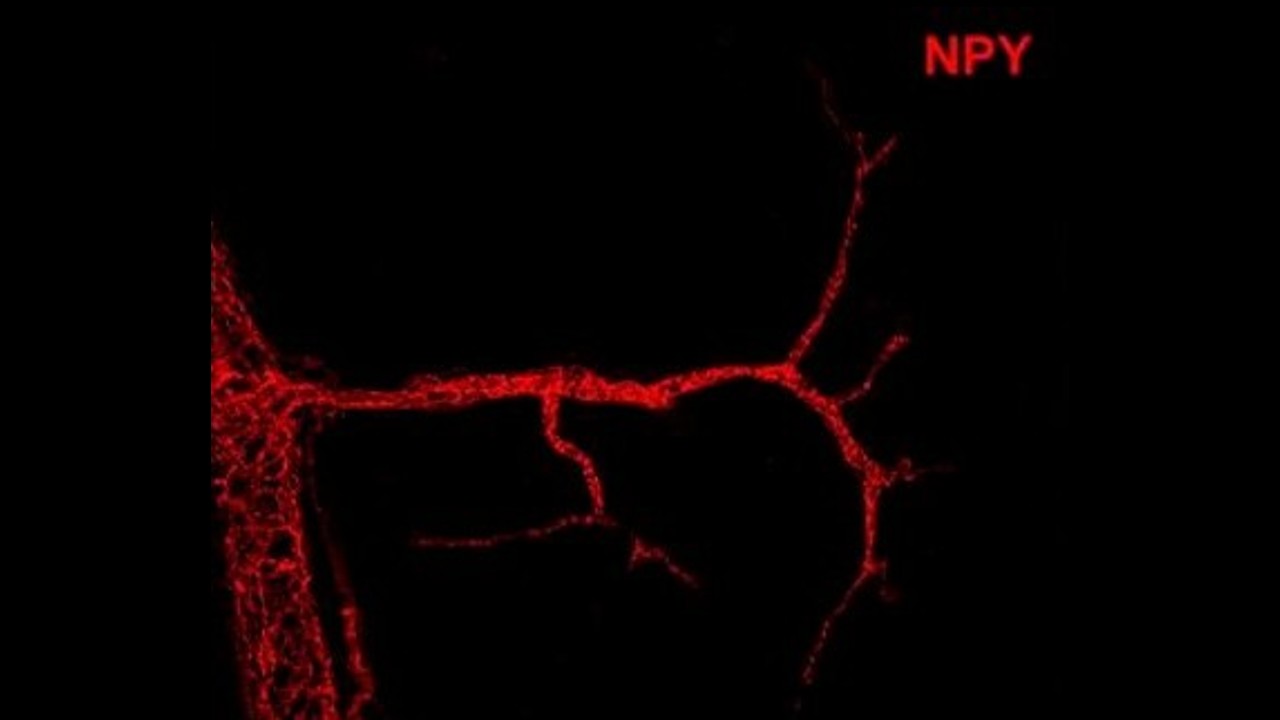
In the image, neuropeptide Y binds to mural cells, precursors of a specific type of adipose tissue that is essential for regulating metabolism (credit: Ana Domingos/Oxford University)
Published on 11/25/2024
By Maria Fernanda Ziegler | Agência FAPESP – An international team of researchers has discovered a new component of the peripheral nervous system that acts by increasing energy metabolism in the body. The finding paves the way for the development of simpler and cheaper drugs to control obesity and weight gain, regardless of the amount of food ingested.
In an article published in the journal Nature, researchers from the University of Oxford in the United Kingdom and the Obesity and Comorbidities Research Center (OCRC) – funded by FAPESP and based at the State University of Campinas (UNICAMP) in Brazil – describe where and how this component of the peripheral nervous system acts through neuropeptide Y (NPY) on energy and calorie expenditure.
This neurotransmitter, which is responsible for passing information between one neuron and another in the brain, has been studied extensively by scientists in relation to the central nervous system, but its action in peripheral nerves (outside the brain and spinal cord) and its ability to act in fat cells (adipocytes) to protect the body against obesity had never been studied.
“Since this new component does not act on the brain, but on the periphery [peripheral nervous system] of the organism, it opens up a new perspective for the development of drugs that can treat obesity more easily, more efficiently and even more cheaply. This is because, unlike the latest anti-obesity drugs that act on the brain, the new therapeutic approach would not need to be a large molecule [biological drug], and much less be able to cross the [blood-brain] barrier that protects the brain, greatly simplifying issues of access and development of a new drug,” says Licio Velloso, principal investigator of the OCRC and one of the authors of the article.
It should be explained that the peripheral nervous system is divided into two major groups: the somatic and the autonomic. While the somatic nervous system regulates voluntary movements and sensations in the skin (touch, cold, heat), the other is responsible for movements such as heartbeat and blood pressure, which function independently of the individual’s will.
The autonomic nervous system is also divided into two parts. The sympathetic branch is responsible for preparing the body for situations of alertness, coping, or fleeing from a threat. It therefore involves changes such as an accelerated heartbeat and increased energy expenditure. The parasympathetic nervous system, on the other hand, normalizes the functioning of the internal organs once the threat has passed.
“Neuroanatomy textbooks, for example, describe the sympathetic nervous system as working by releasing noradrenaline at the nerve terminals. In the study, however, it was found that in addition to noradrenaline, these nerves also express neuropeptide Y, something previously unknown in this part of the nervous system. When NPY is active in the hypothalamus, the individual feels hungry. In the peripheral nervous system, on the other hand, NPY was found to have the opposite effect, accelerating metabolism and, consequently, energy expenditure,” Velloso explains.
Accelerated metabolism
In addition to proving the existence of the new component, Oxford researchers showed that NPY acts on one-third of the sympathetic nerves distributed throughout the body, promoting the production of new fat cells involved in thermogenesis (burning energy and producing heat).
Unlike white adipose tissue, which stores excess calories consumed, brown and beige adipocytes play a metabolically beneficial role by using stored fat to release heat.
In the laboratory of researcher Ana Domingos in Oxford, scientists have discovered that the release of NPY in the peripheral nervous system takes place in synapses that are in contact with mural cells (located around the blood vessels that infiltrate the tissues). “An important feature of mural cells is that they’re precursors of a specific type of adipose tissue, brown adipose tissue, which is essential for regulating metabolism,” Velloso told Agência FAPESP. “In this way, the Oxford research team found that when the nerve connects to a mural cell via a synapse, it releases NPY and activates the differentiation of the mural cell, which becomes a precursor of brown adipose tissue, something very important for greater metabolic control.”
The last part of the study was carried out in the UNICAMP laboratory. To characterize the action of the new peripheral nervous system component, the researchers used mice genetically modified to not express neuropeptide Y in the sympathetic nervous system.
“We observed that these animals became more obese and had difficulty maintaining a stable body temperature when exposed to cold [low thermogenic capacity]. They also had metabolic disorders, such as a predisposition to developing diabetes. On the other hand, the animals with NPY that ate the same amount of food as the others showed protection against obesity,” she says.
Center versus periphery
One curiosity discovered by the group is that NPY has completely different effects on body weight when it is in the central nervous system and when it is in the peripheral nervous system. While in the brain NPY interferes with satiety – inducing hunger – in the periphery its relationship is with energy metabolism, promoting the proliferation of cells that burn fat instead of storing it.
“It was already known that thermogenic adipocytes can originate from mural cells. It was also known that mural cells can detect NPY because they have a receptor for this neuropeptide [the NPYR1 protein]. What we were able to show for the first time is that without NPY in the peripheral system, mice become obese – not because they eat more, but because they burn less fat. This mechanism probably generalizes to other types of obesity, as our study showed that these NPY-producing nerves degenerate with the onset of diet-induced obesity,” Domingos reports.
According to the researcher, the protective role of NPY against obesity now discovered in mice provides a biological mechanism that can be linked to a human genetic trait recently revealed by the Common Metabolic Diseases Knowledge Portal (CMDKP) – a data tool funded by the Accelerating Medicines Partnership (AMP) initiative of the Foundation for the National Institutes of Health (FNIH), a consortium that supports Domingos’ laboratory at the University of Oxford.
The tool, which quantifies the involvement of various genes in diseases, indicated that NPY is associated with human obesity, but not with altered eating patterns.
“This is quite surprising since there are dozens of studies showing the actions of NPY in brain neurons that promote food intake – hence its designation as an appetite stimulant [orexigenic peptide],” she says.
“How, then, could alterations in NPY be paradoxically associated with a high body mass index [BMI] in humans, but not with changes in eating patterns? Our study reveals a possible explanation for this by suggesting that energy dissipation may play a more crucial role than appetite in maintaining body weight in some individuals – although not in most of them,” Domingos concludes.
According to Velloso, the discovery and characterization of this new component of the autonomic nervous system – which regulates metabolism in the body’s periphery – paves the way for the development of new drug therapies for obesity.
“The expectation is that a molecule – probably a ligand for the neuropeptide Y receptor – could act by increasing metabolism, the burning of calories, which is exactly what we found that NPY does in the peripheral nervous system,” she says.
The article “Sympathetic neuron derived NPY protects from obesity by sustaining the mural progenitors of thermogenic adipocytes” can be read at: www.nature.com/articles/s41586-024-07863-6.
Source: https://agencia.fapesp.br/53385