

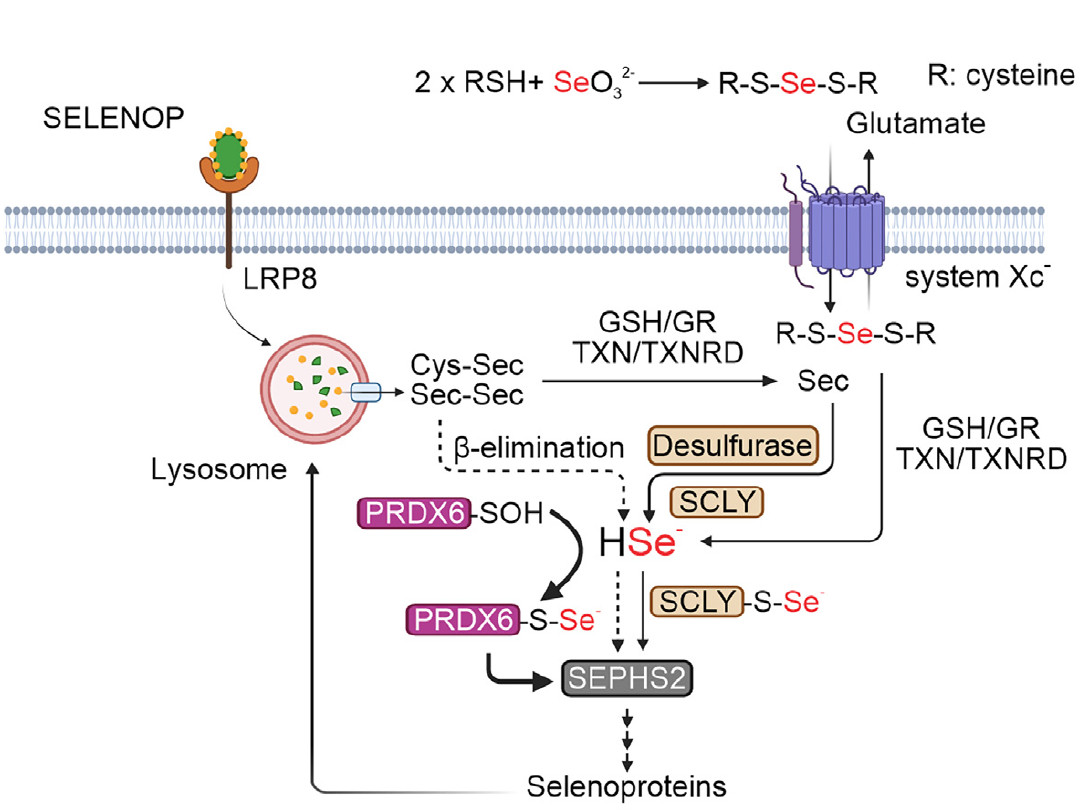
Schematic illustrating the potential role of PRDX6 in regulating selenium metabolism and selenoprotein production (credit: Chen et al./Molecular Cell)
Published on 08/21/2025
Agência FAPESP* – Selenium is a highly important micronutrient for human health. Its biological functions are associated with selenoproteins, which have the amino acid selenocysteine in their structure.
Selenoproteins are generally involved in redox functions in cells (chemical processes involving the transfer of electrons between substances), such as those performed by the vital antioxidant enzyme glutathione peroxidase 4 (GPX4). This enzyme protects membrane lipids and inhibits cell death by ferroptosis (which occurs when iron accumulates inside cells). Thus, selenocysteine metabolism is essential for maintaining cell function and enabling life.
Recently, an international team of researchers announced the discovery of a novel pathway for selenocysteine metabolism mediated by the antioxidant enzyme peroxiredoxin 6 (PRDX6). The study also described an association between high levels of PRDX6 and a highly aggressive subtype of neuroblastoma, suggesting that this mechanism may be exploited to induce ferroptosis in tumor cells.
The results were published as the cover story of the journal Molecular Cell.
According to the authors, the study advances our understanding of selenocysteine metabolism and selenoprotein biosynthesis by revealing a novel function for PRDX6.
“Until recently, it was thought that there was only one pathway for selenocysteine metabolism. However, having parallel pathways is important for a cell, because if, for example, a mutation occurs in selenocysteine lyase [SCLY, an enzyme considered essential for selenocysteine metabolism], the production of selenoproteins would be interrupted, making the cell more sensitive to ferroptosis,” explains Alex Inague, PhD, of the Institute of Chemistry at the University of São Paulo (IQ-USP), in Brazil, and one of the lead authors of the article.
The work was led by José Pedro Friedmann Angeli of the University of Würzburg, in Germany; Sayuri Miyamoto, professor at IQ-USP and member of the Center for Research on Redox Processes in Biomedicine (Redoxoma); and Hamed Alborzinia of the Heidelberg Institute for Stem Cell Technology and Experimental Medicine, in Germany. Redoxoma is a FAPESP Research, Innovation and Dissemination Center (RIDC) based at IQ-USP.
Inague carried out the research during his PhD, under Miyamoto’s supervision and with support from FAPESP, and completed an internship in Angeli’s laboratory.
Ferroptosis and selenoproteins
Ferroptosis is characterized by the accumulation of lipid peroxidation products catalyzed by iron ions, leading to membrane rupture and cell death. “Our cells need fluid membranes so that there’s a regulated mechanism of transport via proteins between the intra- and extracellular media. However, we live in an oxygen-rich environment that generates free radicals and oxidants. The polyunsaturated fatty acids in the membranes are oxidized, forming phospholipid hydroperoxides, which, in the presence of metals, break down to form more lipid radicals. These propagate chain reactions that destabilize the membrane,” explains Miyamoto.
According to the researchers, the mechanisms regulating ferroptosis have attracted increasing interest due to the association of this type of cell death with various pathological conditions, including cancer, neurodegeneration and tissue damage. Induction of ferroptosis may be a promising approach to treating certain cancers, such as neuroblastoma, B-cell lymphoma and undifferentiated melanoma.
In cells, the main defense against ferroptosis is the selenoprotein GPX4, which protects membranes from oxidation. Selenoproteins are rare: only 25 have been identified in the human proteome. Selenocysteine is similar to cysteine, but the sulfur atom is replaced by selenium. This substitution is not straightforward and requires several steps.
“Selenocysteine is encoded by the stop codon UGA, which normally indicates the end of protein synthesis. In the absence of selenium, translation is interrupted and no selenoprotein is produced. Cells regulate this recoding of the stop codon and the expression of selenoproteins through a complex system involving several factors,” explains Inague.
This is because selenide (a chemical compound containing selenium with an oxidation number of -2) is very unstable. “An efficient transport mechanism is needed to get the selenium consumed in the diet to the proteins that are synthesized from it,” adds the IQ-USP professor.
During his research, Inague identified a correlation between PRDX6, enzymes involved in selenium metabolism, and expression levels of selenoproteins such as GPX4. PRDX6 also reduces phospholipid hydroperoxides but, as kinetic studies have shown, at a much slower rate than GPX4.
Using screening techniques and CRISPR/Cas9 gene editing technology in neuroblastoma cell lines, the researchers found that PRDX6 acts independently of the enzyme selenocysteine lyase.
Through a series of experiments with recombinant PRDX6, they demonstrated that PRDX6 can bind to several selenium compounds, suggesting a possible role in selenium transport and an alternative pathway for selenocysteine metabolism.
PRDX6 is highly conserved in evolution and is found in various organisms, from archaea to bacteria to humans. It is found in virtually all organs, particularly the lungs, brain, liver, kidneys and testes.
Cancer and neurodegenerative diseases
Neuroblastoma is a tumor that develops from cells in the nervous system and primarily affects children under the age of 10. Its most aggressive form depends on a selenoprotein P receptor (LRP8) to suppress ferroptosis and proliferation. Given this dependence, the researchers investigated the effect of PRDX6 on neuroblastoma.
To do this, they used a xenograft model, implanting cancer cells from patients into animals to induce tumor growth. The modified cells were then implanted into the rodents’ adrenal glands. Those with PRDX6 and SCLY deletion had fewer tumors and survived longer compared to animals with intact enzymes. This suggests that in the absence of PRDX6 and SCLY, the selenium pathway is compromised, leading to reduced GPX4 expression in tumor cells, which become more susceptible to ferroptosis.
However, it is too early to propose PRDX6 inhibition as a therapeutic approach. According to the authors, further studies are needed to determine whether PRDX6 could serve as a target for drug development.
On the other hand, preventing ferroptosis may also have therapeutic potential. “Evidence suggests that ferroptosis may contribute to the death of motor neurons in amyotrophic lateral sclerosis [ALS]. So it’s a pathway that, if we understand how it happens, we can prevent this death by ferroptosis. There are two sides, we can induce ferroptosis for therapeutic purposes in cancer or prevent ferroptosis and treat a neurodegenerative disease. So we’re trying to do research in both directions,” says Miyamoto.
The article “PRDX6 contributes to selenocysteine metabolism and ferroptosis resistance” is available at: www.cell.com/molecular-cell/fulltext/S1097-2765(24)00867-0.
* With information from Redoxoma
Source: https://agencia.fapesp.br/55666