

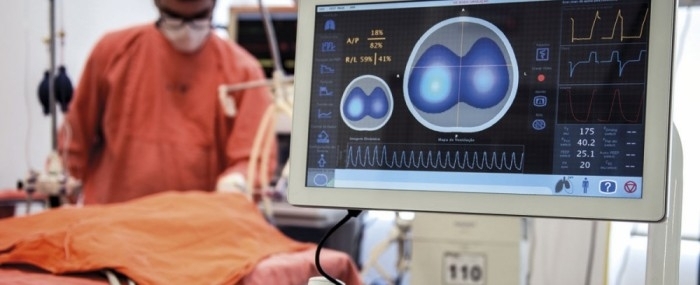
Electrical impedance tomography system developed by startup based in São Paulo minimizes complications associated with mechanical ventilation and is used in the treatment of COVID-19 by hospitals in Italy, Spain and the US (photo: Timpel)
Published on 03/25/2021
By Elton Alisson | FAPESP Innovative R&D – A mechanical ventilation monitoring system using electrical impedance tomography (EIT) developed by Timpel, a startup based on São Paulo, Brazil, could help increase the availability of beds in intensive care units for critical patients with COVID-19.
Developed as part of a project supported by FAPESP, the system is in use at hospitals in Brazil, Europe, the US, Japan and the Middle East. In São Paulo Timpel’s system is used by Hospital das Clínicas, the general hospital run by the University of São Paulo’s Medical School (FM-USP), and also by FM-USP’s Heart Institute (INCOR), the Emílio Ribas Institute (IEER, a major infectious disease hospital), and several private hospitals.
“We currently have more than 150 systems in operation. Some are in use in hospitals in Italy, Spain and the US to treat patients with COVID-19,” said Rafael Holzhacker, CEO of Timpel.
The EIT technology developed by the firm enables healthcare staff to monitor and optimize mechanical ventilation in patients undergoing intensive care so as to minimize adverse side-effects and reduce the amount of time during which they depend on the machine to breathe.
The technological principle behind EIT is resistance to electric current flow (impedance) in the lungs, which varies considerably as the patient breathes in and out.
Fifty images per second are obtained by means of a chest belt with 32 electrodes similar to those used to produce an electrocardiogram. A weak electric current passes through the patient’s chest and highlights the region in which air is circulating as it meets differences in resistance on the way.
The doctor at the bedside thereby receives vital real-time information on the distribution and dynamics of lung insufflation.
Onboard software developed during a project supported by FAPESP via its Innovative Research in Small Business Program (PIPE) enables the medical team to assess the ventilation strategy that bests protects the patient.
“The system provides all the information needed to gauge how the patient is progressing from the bedside, reducing the need for CT scans using X-rays, exposure to radiation and the risk of transport to the radiology suite, which is especially hazardous for COVID-19 patients, who must be kept in isolation,” Holzhacker said.
Adjusting ventilation strategy in this way is particularly important in treating COVID-19 patients, who often need to receive non-intuitive forms of ventilation, he explained. The conventional treatment for patients with very low oxygenation entails a gradual increase in oxygen pressure and concentration, but excessive pressure can damage the lungs, causing volutrauma (alveolar overdistension) and/or barotrauma (rupture of alveoli with subsequent entry of air into the pleural space).
“In these cases the lungs can be damaged as a side-effect of ventilation, in addition to the coronavirus infection,” Holzhacker said.
The firm intends to develop and validate a ventilation strategy algorithm for COVID-19 patients based on data from use of its EIT system in critical cases in Italy and Spain. “The equipment and algorithms in current use are extremely useful for the treatment of COVID-19 patients but we plan to develop an even simpler tool customized for treatment of this disease,” Holzhacker said.
Partnerships with universities
Timpel developed its EIT system through partnerships with the University of São Paulo’s Medical and Engineering Schools, and the Federal University of the ABC (UFABC). The firm’s studies of novel artificial ventilation techniques, dating from 2002, revealed the need for a device to monitor treatment strategies continuously and in an individualized manner.
Its first units were sold in 2015, after the firm won approval from ANVISA, Brazil’s national health surveillance agency, from the European Union, and later from the US Food and Drug Administration (FDA). Initially it was incubated at the Center for Innovation, Entrepreneurship and Technology (CIETEC), an incubator run jointly by the University of São Paulo and the Nuclear and Energy Research Institute (IPEN). It has a branch in the Netherlands with a sales force and clinical staff to support customers in Europe.
Source: https://agencia.fapesp.br/33000